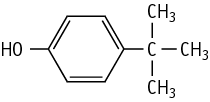

| Purity | : | 99.9 % |
| Test species/strain | : | Mouse/Crj:CD-1 (ICR) |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemincal Substances Control Law of Japan) and OECD Test Guideline 474 |
| Route | : | Intraperitoneal injection |
| Dosage | : | 0 (vehicle), 12.5, 25, 50 mg/kg |
| Number of animals/group | : | Males, 5 |
| Vehicle | : | 0.5 % Methylcellulose |
| Administration period | : | Single |
| Sampling time | : | 24 and 48 hours |
| Positive control | : | Cyclophosphamide |
| Tissue | : | Bone marrow cells |
| GLP | : | Yes |
Test results:
| 1) | The test was performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |