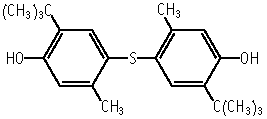

4,4'-Thiobis(6-tert-butyl-m-cresol) was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA, with or without an exogeneous metabolic activation system.
4,4'-Thiobis(6-tert-butyl-m-cresol) induced neither structural chromosomal aberrations nor polyploidy in CHL/IU cells up to concentrations (0.0009-0.02 mg/ml) causing more than 50% cell growth inhibition, in the absence or presence of an exogenous metabolic activation system.
| Purity | : | 98% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 15, 60, 250 mg/kg/day |
| Number of animals/group | : | Males, 6; females, 6 |
| Vehicle | : | 0.5 % gum arabic solution |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Days 29 or 43 |
| GLP | : | Yes |
Test results:
| Purity | : | above 98 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test methods | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD (471 and 472) |
| Procedures | : | Plate incorporation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix, 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, WP2, TA98), Sodium azide (TA1535)and 9-Aminoacridine (TA1537),
+S9 mix, 2-Aminoanthracene (five strains) |
| Doses | : | -S9 mix, 0, 0.781, 1.56, 3.13, 6.25, 12.5, 25 and 50 μg/plate (TA100, TA1535 and TA1537), 0, 3.13 - 200 μg/plate (WP2) and 0, 313 - 5000 μg/plate (WP2)
+S9 mix, 0, 12.5, 25, 50, 100, 200, 400 and 800 μg/plate (TA100, TA1535, TA98 and TA1537) and 0, 313 - 5000 μg/plate (WP2) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | more than 98% |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Dimethylsulfoxide |
| Positive controls | : | -S9 mix, Mitomycin C
+S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix (continuous treatment): 0, 0.00050, 0.0010,0.0020 mg/ml
-S9 mix (short-term treatment): 0, 0.00023, 0.00045, 0.00090 mg/ml +S9 mix (short-term treatment): 0, 0.0050, 0.010,0.020 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Bozo Research Center Inc, 1284, Kamado, Gotemba-shi, Shizuoka, 412, Japan. Tel +81-550-82-2000 Fax +81-550-82-2379 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |