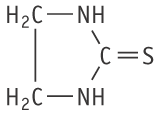
Molecular formula: C3H6N2S Molecular weight: 102.16

2-Imidazolidinethione was administered repeatedly by oral gavage to male and female Crj:CD(SD)IGS rats at dose levels of 0, 1, 6, and 30 mg/kg for 28 days, and its toxicity was examined.
Changes attributable to the test substance were apparent in clinical signs, body weights, food consumption, blood chemistry, organ weights and necropsy findings, and on histopathological examination of both sexes of the 6 and 30 mg/kg groups.
On observation of clinical signs, abnormal fur (loss of gloss) was noted consistently in both sexes of the 30 mg/kg group. During the recovery period, although not completely this gradually disappeared.
Decreases in body weight and food consumption were observed in both sexes of the 30 mg/kg group, but disappeared by the final week of the recovery period.
The following changes in blood chemistry were observed in the 30 mg/kg group; increases in total cholesterol as well as decreases in ALP and inorganic phosphorus among males, and increases in chloride in both sexes. These changes had disappeared by the end of the recovery period.
On measurement of organ weights, the following changes were observed; increases in relative liver weights in females of the 30 mg/kg group, increases in absolute and relative thyroid weights in both sexes of the 30 mg/kg group, and decreases in absolute and relative thymus weights in females of the 6 and 30 mg/kg groups. These changes disappeared or were alleviated by the end of the recovery period.
At necropsy, enlargement of the thyroids was observed in males of the 6 mg/kg group and in both sexes of the 30 mg/kg group. This change disappeared or was alleviated by the end of the recovery period.
On histopathological examination, centrilobular hypertrophy of hepatocytes, basophilic hypertrophy in the anterior lobe in the pituitary, and atrophy of sebaceous glands were observed in both sexes of the 30 mg/kg group. Furthermore, decreases in colloid in the thyroid and diffuse hypertrophy of follicular cells were observed in 6 mg/kg males and both sexes of the 30 mg/kg group. Although basophilic hypertrophy of the anterior lobe in the pituitary were still seen in males of the 30 mg/kg group at the end of the recovery period, it gradually disappeared. Moreover, the other changes had disappeared by the end of the recovery period.
There were no changes considered attributable to the test substance in the functional observation battery, hematology, or urinalysis.
In summary, significant changes considered attributable to the test substance were found in both sexes of the 6 mg/kg or higher groups. The NOEL is considered to be 1 mg/kg/day for both males and females under the conditions of this study.
2-Imidazolidinethione proved mutagenic in Salmonella typhimurium TA1535, with or without an exogenous metabolic activation system.
2-Imidazolidinethione did not induce structural chromosomal aberrations or polyploidy in CHL/IU cells with or without exogenous metabolic activation.
| Purity | : | 99.89 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | Guideline for the 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral(gavage) |
| Dosage | : | 0(vehicle), 1, 6, 30 mg/kg/day |
| Number of animals/group | : | Males, 10; females, 10(0, 30 mg/kg) Males, 5; females, 5(1, 6 mg/kg) |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose solution |
| Dosing period | : | 28 days |
| Terminal killing | : | Day 29 or 43 |
| GLP compliance | : | Yes |
Test results
Changes attributable to the test substance were apparent in clinical signs, body weights, food consumption, blood chemistry, organ weights and necropsy findings and on histopathological examination of both sexes of the 6 and 30 mg/kg groups.
As a clinical sign, abnormal fur (loss of gloss) was observed in both sexes of the 30 mg/kg group, starting from day 6, and increasing thereafter. In the 1 and 6 mg/kg groups, no abnormalities were observed. During the recovery period, reduction of affected areas was apparent, suggestive of reversibility.
In the dosing period, decreased body weights were observed in both sexes of the 30 mg/kg group from day 15. In contrast, no changes were observed in males or females of the 1 and 6 mg/kg groups. In the recovery period, male body weight gain then returned almost to the level of the control group and this was complete in females of the 30 mg/kg group.
In the dosing period, decreased food consumption was observed in males of the 30 mg/kg group from day 8 and in females on day 15; however, food consumption recovered by the final week (day 39).
In the blood chemistry, the following changes were observed; increases in chloride in both sexes of the 30 mg/kg group, increase in total cholesterol as well as decreases in ALP and inorganic phosphorus in males of the 30 mg/kg group, and increase in g-GT in females of the 30 mg/kg group. These changes were no longer apparent at the end of the recovery period.
The following changes in organ weights were observed; increases in absolute and relative thyroid weights in both sexes of the 30 mg/kg group, increases in relative liver weights in females of the 30 mg/kg group, and decreases in absolute and relative thymus weights in females of the 1, 6 and 30 mg/kg groups. At the end of the recovery period, increases in relative thyroid weights were still observed in females of the 30 mg/kg group; however, their degree was reduced. The other changes disappeared by the end of the recovery period.
At necropsy, treatment-related changes were observed in the thyroids and skin of both sexes. In the animals necropsied at the end of the dosing period, enlargement of the thyroids was observed in 1 male of the 6 mg/kg group and 4 males and 2 females of the 30 mg/kg group. The changes observed in 1 male of the 6 mg/kg group and 1 female of the 30 mg/kg group were unilateral. In addition, loss of gloss in fur was observed in all males and 4 females of the 30 mg/kg group. The changes were seen consistently and were most remarkable in the interscapular region. In the animals necropsied at the end of the recovery period, the thyroids no longer were abnormal and fur had also almost returned to normal. The number of affected animals was fewer than at the end of the dosing period and lesions were only focal on the head, chest, abdomen, and lumbar region.
As histopathological findings, treatment-related changes were observed in the liver, pituitary, thyroids and skin. Increases in centrilobular hypertrophy of hepatocytes were observed in both sexes of the 30 mg/kg group at the end of the dosing period. This was no longer observed in the animals necropsied at the end of the recovery period. Basophilic hypertrophy of the anterior lobe in the pituitary was noted in all males and 2 females of the 30 mg/kg group. This change was severe in all males and there were large vacuoles in cytoplasm of many of the hypertrophic cells. In the animals necropsied at the end of the recovery period, similar changes were observed in 3 males of the 30 mg/kg group; however, they were slight. Decreases in colloid in the thyroid were apparent in 4 males of the 6 mg/kg group and was severe in all males and most females of the 30 mg/kg group. In all animals demonstrating decreases in colloid in the thyroid, diffuse hypertrophy of follicular cell in the thyroid was observed, the extents of the two changes correlating. They were no longer observed in the animals necropsied at the end of the recovery period. Atrophy of sebaceous gland in the skin was noted in all males and 3 females of the 30 mg/kg group necropsied at the end of the dosing period. This change was considered to correspond to the loss of gloss in fur. However, atrophy of sebaceous gland in the skin was not apparent in 1 male with the loss of gloss. Furthermore, atrophy of sebaceous glands in the skin was no longer seen at the end of the recovery period despite the partial persistence of abnormal fur.
There were no changes considered attributable to the test substance in the functional observation battery, hematology or urinalysis.
The no-observed-effect level (NOEL) is considered to be 1 mg/kg/day for both males and females under the conditions of this study.
| Purity | : | 99.89 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA/pKM101 |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, WP2 uvrA/pKM101, TA98), sodium azide (TA1535) and 9-aminoacridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (all strains) +S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (all strains) |
| S9 | : |
Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : |
3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Salmonella typhimurium TA1535
| + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] |
| With metabolic activation: | [*] | [ ] | [ ] |
Escherichia coli WP2 uvrA/pKM101
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.89 % |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Physiological saline |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Benzo[a]pyrene |
| Dosage | : | -S9 mix(6 hr short-term treatment); 258, 515, 1030 μg/mL +S9 mix(6 hr short-term treatment); 258, 515, 1030 μg/mL -S9 mix(24 hr continuous treatment); 258, 515, 1030 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
This chemical did not induce structural chromosomal aberrations or polyploidy under the conditions of this experiment.
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Kashima Laboratory, Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki 314-0255, Japan. Tel +81-479-46-2871, Fax +81-479-46-2874. |