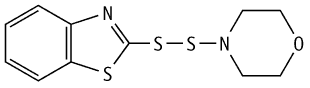
2-(4-Morpholinyldithio)benzothiazole was studied for oral toxicity in rats in a 28-day repeated dose toxicity test at doses of 0, 100, 300, and 1000 mg/kg/day.
Neither dead nor moribund animals were noted in either sex in any group. No changes attributable to the test substance were noted in general signs or body weights. Transiently low food consumption and transiently high water intake were noted in both sexes at 1000 mg/kg. In the organ weight measurement, the absolute liver weights tended to be high, and the relative liver weights were high in the males given 1000 mg/kg and in the females receiving 300 or 1000 mg/kg. No changes in liver weight were noted in either sex at the end of the recovery period. No changes attributable to the test substance were noted in behavior, sensory response, grip strength, spontaneous motor activity, urinalysis, hematological examination, blood chemical analysis, necropsy findings, or histopathological findings.
The NOELs for repeated dose toxicity are considered to be 300 mg/kg/day for males and 100 mg/kg/day for females.
2-(4-Morpholinyldithio)benzothiazole was studied for oral toxicity in rats in an OECD preliminary reproduction toxicity screening test at doses of 0, 100, 300, and 1000 mg/kg/day.
Neither dead nor moribund animals were noted in either sex in any group. No changes attributable to the test substance were noted in general signs. Male body weights were low, and female body weights were low during the pregnancy period at 1000 mg/kg. Transiently low food consumption was noted in both sexes at 300 mg/kg and in the males at 1000 mg/kg, and low food consumption was noted in the females at 1000 mg/kg. No changes attributable to the test substance were apparent in either sex in necropsy findings, organ weights, or histopathological findings. Also, no changes attributable to the test substance were noted in sperm analysis. The NOEL for repeated dose toxicity is considered to be 100 mg/kg/day for both sexes.
Regarding the reproductive and developmental toxicity, no changes attributable to the test substance were noted in parental animals or pups. The NOEL for reproductive performance is considered to be 1000 mg/kg/day for parental animals and pups.
Reverse mutation tests using microorganisms (Salmonella typhimurium, Escherichia coli) were conducted to assess the potential of 2-(4-morpholinyldithio)benzothiazole to induce gene mutations. 2-(4-Morpholinyldithio)benzothiazole did not induce gene mutations in the bacteria under the conditions of this study.
In vitro chromosomal aberration tests using cultured Chinese hamster cells (CHL/IU) were conducted to assess the potential of 2-(4-morpholinyldithio)benzothiazole to induce chromosomal aberrations. 2-(4-Morpholinyldithio)benzothiazole induced chromosomal aberrations in cultured cells under the conditions of this study.
| Purity | : | 98.4 % |
| Test Species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose solution |
| GLP | : | Yes |
Test results:
| Purity | : | 98.4 % |
| Test Species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 407 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 100, 300, 1000 mg/kg |
| Number of animals/group | : | Males, 12; females, 12 (0, 300, 1000 mg/kg) Males, 6; females, 6 (100 mg/kg) |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose solution |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Males and females, day 29 or 43 |
| GLP | : | Yes |
Test results:
The NOELs for repeated dose toxicity are considered to be 300 mg/kg/day for males and 100 mg/kg/day for females.
| Purity | : | 98.4 % |
| Test Species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 100, 300, 1000 mg/kg |
| Number of animals/group | : | Males, 12; females, 12 |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose solution |
| Administration period | : | Males, 50-52 days Females, from 14 days before mating to day 3 of lactation |
| Terminal killing | : | Males, days 51-53 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
Neither dead nor moribund animals were noted in either sex in any group. No changes attributable to the test substance were noted in general signs. Male body weights were low, and female body weights were low during the pregnancy period at 1000 mg/kg. Transiently low food consumption was noted in both sexes at 300 mg/kg and in the males at 1000 mg/kg, and low food consumption was noted in the females at 1000 mg/kg. No changes attributable to the test substance were noted in either sex for necropsy findings, organ weights, or histopathological findings. Also, no changes attributable to the test substance were noted on sperm analysis.
The NOEL for repeated dose toxicity is considered to be 100 mg/kg/day for both sexes.
<Reproductive and Developmental Toxicity>
Regarding the reproductive and developmental toxicity, no changes attributable to the test substance were noted in terms of the number of estrous cases, copulation index, number of conceiving days, fertility index, length of gestation, gestation index, delivery conditions, nursing conditions, number of corpora lutea, number of implantation sites, or the implantation index.
The NOEL for reproductive performance of parental animals is considered to be 1000 mg/kg/day for both sexes.
Regarding the pups, no changes attributable to the test substance were noted in terms of their number, the numbers of stillbirths, and live pups on Day 0 of lactation, sex ratio, delivery index, birth index, live birth index, general signs, number of live pups on Day 4 of lactation, viability index on Day 4 of lactation, external features, body weight change, or necropsy findings.
The NOEL for pups is considered to be 1000 mg/kg/day.
| Purity | : | 98.4 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA), Sodium azide (TA1535) and 9-Amino-acridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate (all strains for the dose-finding study) +S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate (all strains for the dose-finding study) -S9 mix; 0, 2.34, 4.69, 9.38, 18.8, 37.5, 75.0, 150 μg/plate (all strains for the main study) +S9 mix; 0, 18.8, 37.5, 75.0, 150, 300, 600 μg/plate (TA100 for the main study) +S9 mix; 0, 4.69, 9.38, 18.8, 37.5, 75.0, 150, 300 μg/plate (TA1535, WP2 uvrA, TA98, TA1537 for the main study) -S9 mix; 0, 2.34, 4.69, 9.38, 18.8, 37.5, 75.0, 150 μg/plate (all strains for the additional study) +S9 mix; 0, 4.69, 9.38, 18.8, 37.5, 75.0, 150, 300 μg/plate (TA100 for the additional study) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 3 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 98.4 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 14.0, 23.3, 38.9 μg/mL (main study) +S9 mix (short-term treatment); 0, 38.9, 64.8, 108 μg/mL (main study) -S9 mix (short-term treatment); 0, 20.0, 30.0, 40.0 μg/mL (confirmation study) +S9 mix (short-term treatment); 0, 80.0, 90.0, 100, 110, 120 μg/mL (confirmation study) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| Lowest concentration producing cytogenetic effects in vitro <Confirmation test> | : | ||
| With metabolic activation (short-term treatment) | : | 80.0 μg/mL (clastogenicity) | |
Genetic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [*] | [ ] | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Nihon Bioresearch Inc., 6-104, Majima, Fukuju-cho, Hashima, Gifu, 501-6251, Japan. Tel +81-58-392-6222 Fax +81-58-392-1284 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides (An-pyo Center), 582-2 Shioshinden, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |