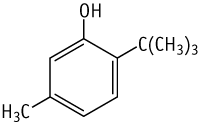

Single oral dose toxicity test of 6-tert-butyl-m-cresol in rats revealed LD50 values between 320 and 800 mg/kg in males and between 130 and 320 mg/kg in females.
An OECD combined repeat dose and reproductive/developmental toxicity screening test was performed in rats for 6-tert-butyl-m-cresol. With regard to repeat dose toxicity, no animal died in any groups at 2.5, 12.5 and 60 mg/kg/day of the test substances. Suppression of body weight and decrease in food consumption were observed in females of the 60 mg/kg group, and increase in the kidney weight along with liver weight increase. Hypertrophy of centrilobular hepatocytes were observed in both sexes given 60 mg/kg. In the 2.5 and 12.5 mg/kg groups, no adverse effects of 6-tert-butyl-m-cresol were observed on organ weights and no histopathological changes were found. Treatment did not affect the general condition in males and females, and body weight gain, food consumption and hematological parameters in males. The NOEL for repeat dose toxicity is considered to be 12.5 mg/kg/day in males and females.
Regarding reproductive and developmental toxicity, no adverse effects were observed on copulation, fertility, delivery and lactation in any groups. Slight decrease in number of corpora lutea, implants, number of live neonates at birth, delivery index and live birth index were observed in the 60 mg/kg group. Suppression of body weight gain of pups was found in this group. Administration of 6-tert-butyl-m-cresol did not affect the sex ratio and morphological appearance of pups at any dose level. NOELs for reproductive and developmental toxicity are considered to be 60 mg/kg/day in males, 12.5 mg/kg/day in females, and 12.5 mg/kg/day in pups, respectively.
6-tert-Butyl-m-cresol was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
6-tert-Butyl-m-cresol induced structural chromosomal aberrations in CHL cells after short-term treatment with an exogenous metabolic activation system.
| Purity | : | 99.23 % |
| Test species/strain | : | Rat/Crj:CD(SD) |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavege) |
| Doses | : | 0, 130, 320, 800, 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Corn oil |
| GLP | : | Yes |
Test results:
Decrease in body weight was noted in the males receiving 320 mg/kg or more and with 800 mg/kg and more in females. Pathological lesions due to 6-tert-butyl-m-cresol were observed in the digestive organ and kidney.
The LD50 values were between 320 and 800 mg/kg in males and between 130 and 320 mg/kg in females.
| Purity | : | 99.23 % |
| Test species/strain | : | Rat/Crj:CD(IGS) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 2.5, 12.5, 60 mg/kg/day |
| Number of animals/group | : | Males, 13; females, 13 |
| Vehicle | : | Corn oil |
| Administration period | : | Males, 42 days Females, from 14 days prior to mating to day 3 of lactation |
| Terminal killing | : | Males, day 43 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
No animal died in any group. Suppression of body weight and decrease in food consumption were observed in females of the 60 mg/kg group, along with liver weight increase. Hypertrophy of centrilobular hepatocytes was observed in males and females of the 60 mg/kg group. Whereas an increase in the kidney weight was also found in both sexes given 60 mg/kg, no adverse effects of 6-tert-butyl-m-cresol were detected on histopathological examination of the kidneys nor in an urinary analysis for males. In the 2.5 and 12.5 mg/kg groups, no adverse effects of 6-tert-butyl-m-cresol were observed in terms of organ weights or histopathological changes. Treatment with 6-tert-butyl-m-cresol did not affect the general condition in males and females, and body weight gain, food consumption and hematological parameters in males.
The no observed effect dose level (NOEL) for repeat dose toxicity is considered to be 12.5 mg/kg/day in males and females.
<Reproductive and developmental toxicity>
No adverse effects of 6-tert-butyl-m-cresol were observed on copulation, fertility, delivery and lactation in any group. Slight decrease in number of corpora lutea, implants, number of live neonates at birth, delivery index and live birth index were observed in the 60 mg/kg group. Suppression of body weight gain of pups was found in this group. Administration of 6-tert-butyl-m-cresol did not affect the sex ratio and morphological appearance of pups at any dose level.
NOELs for reproductive and developmental toxicity are considered to be 60 mg/kg/day in males, 12.5 mg/kg/day in females, and 12.5 mg/kg/day in pups, respectively.
| Purity | : | 99.23 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD Test Guidelines 471 and 472 |
| Procedures | : | Pre-incubation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98; WP2 uvrA), Sodium azide (TA1535) and 9-Aminoacridine (TA1537) +S9 mix; 2-Aminoanthracene(all strains) |
| Doses | : | -S9 mix; 0, 6.25, 12.5, 25, 50, 100, 200 μg/plate +S9 mix; 0, 6.25, 12.5, 25, 50, 100, 200 μg/plate |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.23 % |
| Type of cell used | : | Chinese hamster lung (CHL) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD Test Guideline 473 |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix, 1-methyl-3-nitro-1-nitrosoguanidine +S9 mix, Benzo[a]pyrene |
| Doses | : | -S9 mix (24 and 48 hr continuous treatment); 0, 10, 20, 40, 60, 80 μg/mL -S9 mix (6 hr short-term treatment); 0, 7.5, 15, 30, 60, 90, 120 μg/mL +S9 mix (6 hr short-term treatment); 0, 7.5, 15, 30, 60, 90, 120 μg/mL [Confirmative test] -S9 mix (6 hr short-term treatment); 0, 2.5, 5, 7.5, 10, 15, 30 μg/mL +S9 mix (6 hr short-term treatment); 0, 2.5, 5, 7.5, 10, 15, 30 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Cytotoxicity was observed at 80 μg/mL after 24 and 48 hr continuous treatment without an S9 mix, and at 120 μg/mL in the 6 hr short-term teatment with and without an S9 mix.
| Lowest concentration producing cytogenetic effects in vitro: | ||
| With metabolic activation (6 hr short-term treatment) | : | 7.5 μg/mL (clastogenicity) |
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |
| 2) | The test were performed by the Research Institute for Animal Science in Biochemistry and Toxicology, 3-7-11 Hashimotodai, Sagamihara-shi, Kanagawa, 229-1132, Japan. Tel +81-42-762-2775 Fax +81-42-762-7979 |