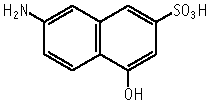

7-Amino-4-hydroxy-2-naphthalenesulfonic acid was mutagenic to Salmonella typhimurium, TA100, TA98, TA1535 and TA1537 with an exogeneous metabolic activation system.
7-Amino-4-hydroxy-2-naphthalenesulfonic acid induced neither structural chromosomal aberrations nor polyploidy in CHL/IU cells, in the absence or presence of an exogenous metabolic activation system.
| Purity | : | 91.8% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-day Repeat Dose Toxicity Testing of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 250, 500, 1000 mg/kg/day |
| Number of animals/group | : | Males, 6 and 12 (0, 1000 mg/kg) Females, 6 and 12 (0, 1000 mg/kg) |
| Vehicle | : | 5% gum arabic solution |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Days 29 or 43 |
| GLP | : | Yes |
Test results:
| Purity | : | 91.8% |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537, E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Pre-incubation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix;AF-2 (TA100, TA98 and WP2 uvrA), Sodium azide (TA1535), 9-Aminoacridine (TA1537) +S9 mix;2-Aminoanthracene (all strains) |
| Doses | : | 156, 313, 625, 1250, 2500, 5000 μg/plate |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Genetic effects:
S. typhimurium TA100, TA1535, TA98 and TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] |
E. coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [*] | [ ] |
| Purity | : | 91.8% |
| Type of cell used | : | Chinese hamster lung (CHL) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | DMSO |
| Positive controls | : | long-term treatment; Mitomycin C (24 h and 48 h) short-term treatment; Cyclophosphamide (+S9 and -S9 mix) |
| Doses | : | long-term treatment; 0, 375, 750, 1500 μg/ml (24 h and 48 h) short-term treatment; 0, 375, 750, 1500 μg/ml (+S9 and -S9 mix) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Safety Assessment Laboratory, Panapharm Laboratories Co., Ltd. 1285 Kurisaki-machi, Uto-shi, Kumamoto, 869-04, Japan. Tel +81-964-23-5111 Fax +81-964-23-2282 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides (An-pyo Center), Japan, 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka, 437-12, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |