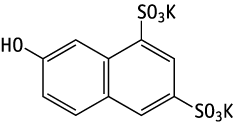

Potassium 7-hydroxy-1,3-naphthalenedisulfonate was studied for oral toxicity in rats in a 28-day repeat dose toxicity test at doses of 0, 100, 300 and 1000 mg/kg/day.
Hematological examination revealed increase in hematocrit, hemoglobin and RBCs, and a decrease in reticulocytes in females given 1000 mg/kg. Blood chemical examination revealed a decrease in total cholesterol in females given 300 and 1000 mg/kg. Absolute and relative spleen weights were decreased in males given 1000 mg/kg.
The NOELs for repeat dose toxicity are considered to be 300 mg/kg/day for males, and 100 mg/kg/day for females.
| Purity | : | 89.2 % |
| Test species/strain | : | Rat/Crj:CD(SD) |
| Test method | : | Guideline for 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral(gavage) |
| Doses | : | 0(vehicle), 100, 300, 1000 mg/kg Number of animals/group |
| Administration period | : | Males and females; 14, 7, 7 and 14/group for the 0, 100, 300 and 1000 mg/kg, respectively |
| Recovery period | : | Males and females; 7 and 7/group for the 0 and 1000 mg/kg cases, respectively |
| Vehicle | : | 1 % Carboxymethylcellulose sodium solution |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Days 29 and 43 |
| GLP | : | Yes |
Test results:
The NOELs for repeat dose toxicity are considered to be 300 mg/kg/day for males, and 100 mg/kg/day for females.
| 1) | The test was performed by the Safety Research Institute for Chemical Compounds Co., Ltd., 363-24, Shin-ei, Kiyota-ku, Sapporo, Hokkaido, 004-0839, Japan. Tel +81-11-885-5031 Fax +81-11-885-5313 |