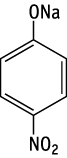

A single dose oral toxicity test of p-nitrophenol sodium salt revealed an LD50 value of 550 mg/kg for males and 467 mg/kg for females.
ρ-Nitrophenol sodium salt was studied for oral toxicity in rats in a 28-day repeat dose toxicity test at doses of 0, 60, 160, 400 and 1000 mg/kg.
All males and females given 1000 mg/kg died from some affects of the test article on the central nerve system without obvious organic change. In the survivors, decrease in spontaneous movement, oligopnea and adoption of a prone/lateral position were observed in both sexes in the 1000 mg/kg group in the early stage of the administration period. A decrease in urine pH was observed in males in the 400 mg/kg group. Histopathological examination in the kidneys revealed an increased incidence of eosinophilic bodies in tubular epithelium in males in the 400 and 1000 mg/kg groups. All the changes had disappeared after a 14-day recovery period. The NOELs are considered to be 160 mg/kg/day for males and 400 mg/kg /day for females.
Genotoxicity of p-nitrophenol sodium salt was studied by reverse mutation test in bacteria and chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells. ρ-Nitrophenol sodium salt was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
ρ-Nitrophenol sodium salt induced structural chromosomal aberrations in CHL/IU cells after short-term treatment, with and without an exogenous metabolic activation system.
| Purity | : | 79.4 % |
| Test species/strains | : | Rat/Crj:CD(SD) |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Doses | : | 0(vehicle), 125, 250, 500, 1000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | 0.5 w/v% Carmellose sodium solution |
| GLP | : | Yes |
Test results:
| Purity | : | 79.4 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | Guideline for 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral(gavage) |
| Doses | : | 0(vehicle), 60, 160, 400, 1000 mg/kg/day |
| Number of animals/group | : | Males, 6; females, 6 |
| Vehicle | : | 0.5 w/v% Carmellose sodium solution |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Males and females, on days 29 and 43 |
| GLP | : | Yes |
Test results:
| Purity | : | 79.4 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals(Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA), Sodium azide (TA1535) and
9-Aminoacridine (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Doses | : | -S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate +S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98 and TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 79.4 % |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemical (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix; 1-Methyl-3-nitro-1-nitrosoguanidine +S9 mix; 3,4-Benzo[a]pyrene |
| Doses | : | -S9 mix(6 hr short-term treatment); 0, 100, 200, 400, 800, 1200, 1600 μg/mL +S9 mix(6 hr short-term treatment); 0, 600, 800, 1000, 1200, 1400, 1600 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Cytotoxicity was observed at 1600 μg/mL without S9 mix, and at 1400 and 1600 μg/mL with S9 mix on 6 hr short-term treatment.
| Lowest concentration producing cytogenetic effects in vitro: | ||
| With metabolic activation (6 hr short-term treatment) | : | 600 μg/mL(clastogenicity) |
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Bozo Research Center Inc, 1284, Kamado, Gotemba-shi, Shizuoka, 412-0039, Japan. Tel +81-550-82-2000 Fax +81-550-82-2379 |
| 2) | The tests were performed by the Research Institute for Animal Science in Biochemistry and Toxicology, 3-7-11 Hashimotodai, Sagamihara-shi, Kanagawa 229-1132, Japan. Tel +81-42-762-2775 Fax +81-42-762-7979 |