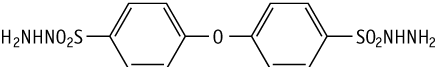
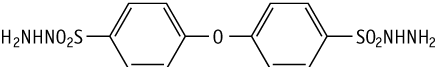
With regard to the single dose toxicity, one male dosed with 1500 mg/kg and 2 animals of each sex dosed with 2000 mg/kg died after showing paralytic or tiptoe gait and/or soiling of fur.
The LD50 value was estimated to be more than 2000 mg/kg for both sexes.
With regard to repeat dose toxicity, decrease of grip strength, a paralytic gait, degeneration of peripheral nerve fibers, increase of kidney weights, fatty degeneration of proximal tubular epithelium, and shortening of prothrombin time were observed in animals receiving 30 mg/kg/day or more. An anemic condition, decrease of body weight and food consumption, increase of total cholesterol and liver weight, and hypertrophy of centrilobular hepatocytes were observed in animals receiving 100 mg/kg or more per day. Death following paralytic gait, diarrhea, decrease of body weight and food consumption was observed in some animals receiving 200 mg/kg/day. Others receiving this dose were killed in a moribund condition.
The NOEL is considered to be 10 mg/kg/day for both sexes.
With regard to the preliminary reproduction toxicity, increase of liver and/or kidney weights was observed in parent animals receiving 10 mg/kg/day or more, but no effects were observed on the reproductive performance of the parents or on the developmental performance in the pups.
The NOELs for systemic toxicity in the parents and reproductive and developmental toxicity are considered to be 3 mg kg/day, and 30 mg/kg/day respectively.
Reverse mutation tests using microorganisms (Salmonella typhimurium, Escherichia coli) were conducted to assess the potential of 4,4'-oxybis(benzenesulfonylhydrazide) to induce gene mutations. 4,4'-Oxybis(benzenesulfonylhydrazide) induced gene mutations in TA100, TA1535 and WP2 uvrA under the conditions of this study.
In vitro chromosomal aberration tests using cultured Chinese hamster cells (CHL/IU) were conducted to assess the potential of 4,4'-oxybis(benzenesulfonylhydrazide) to induce chromosomal aberrations. 4,4'-Oxybis(benzenesulfonylhydrazide) induced chromosomal aberrations in cultured cells under the conditions of this study.
| Purity | : | 99.3 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 1000, 1500, 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Corn oil |
| GLP | : | Yes |
Test results:
The LD50 value was estimated to be more than 2000 mg/kg for both sexes.
| Purity | : | 99.3 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 407 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 1000, 1500, 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Corn oil |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Males and females, days 29 or 43 |
| GLP | : | Yes |
Test results:
The NOEL is considered to be 10 mg/kg/day for both sexes.
| Purity | : | 99.3 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 3, 10, 30 mg/kg |
| Number of animals/group | : | Males, 10; females, 10 |
| Vehicle | : | Corn oil |
| Administration period | : | Males, 46 days Females, from 14 days before mating to day 4 of lactation |
| Terminal killing | : | Males, day 47 of the administration Females, day 5 of lactation |
| GLP | : | Yes |
Test results:
The NOEL for systemic toxicity in parents is considered to be 3 mg/kg/day, and that for reproductive and developmental toxicity is considered to be 30 mg/kg/day.
| Purity | : | 99.3 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA), Sodium azide (TA1535) and 9-Amino-acridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate (all strains for the dose-finding study) +S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate (all strains for the dose-finding study) -S9 mix; 0, 4.88, 9.77, 19.5, 39.1, 78.1, 156 μg/plate (TA100 for the main study) -S9 mix; 0, 2.44, 4.88, 9.77, 19.5, 39.1, 78.1 μg/plate (TA1535 for the main study) -S9 mix; 0, 39.1, 78.1, 156, 313, 625, 1250 μg/plate (WP2 uvrA for the main study) -S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (TA98, TA1537 for the main study) +S9 mix; 0, 4.88, 9.77, 19.5, 39.1, 78.1, 156 μg/plate (TA100 for the main study) +S9 mix; 0, 0.610, 1.22, 2.44, 4.88, 9.77, 19.5 μg/plate (TA1535 for the main study) +S9 mix; 0, 39.1, 78.1, 156, 313, 625, 1250 μg/plate (WP2 uvrA for the main study) +S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (TA98, TA1537 for the main study) -S9 mix; 0, 439, 658, 988, 1481, 2222, 3333, 5000 μg/plate (TA1537 for the confirmation study) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 3 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535
| + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] |
| With metabolic activation: | [*] | [ ] | [ ] |
Salmonella typhimurium TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] |
| With metabolic activation: | [*] | [ ] | [ ] |
| Purity | : | 99.3 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 557, 696, 870, 1088 μg/mL +S9 mix (short-term treatment); 0, 357, 446, 557 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| Lowest concentration producing cytogenetic effects in vitro | : | ||
| Without metabolic activation(short-term treatment) | : | 557 μg/mL (clastogenicity) | |
| With metabolic activation (short-term treatment) | : | 446 μg/mL (clastogenicity) | |
Genetic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Safety Research Institute for Chemical Compounds Co., Ltd., 363-24 Shin-ei, Kiyota-ku, Sapporo, Hokkaido, 004-0839, Japan. Tel +81-11-885-5031 Fax +81-11-885-5313 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), 582-2 Shioshinden, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |