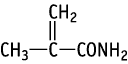
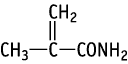
With 200 mg/kg, one out of 13 males and four out of 13 females were found dead and one female was sacrificed on becoming moribund. Dragging of hindlimbs appeared in all animals of the 200 mg/kg group. Body weight gain was decreased in the 50 and 200 mg/kg groups. Food consumption was also decreased in males given 50 and 200 mg/kg, and in females receiving 200 mg/kg. Although inflammation of the lung was observed at 200 mg/kg, the reproductive organs were not affected in either sex. Fertility and estrous cyclicity were not altered, but the copulation rate was decreased at 200 mg/kg. Delayed parturition and abnormal nursing were observed at 200 mg/kg. Lower body weights and decreased viability of the pups were observed at 200 mg/kg. No morphological abnormalities were found in any pups.
In conclusion, the NOEL for systemic toxicity was considered to be 12.5 mg/kg/day in male and female rats, and that for reproductive and developmental toxicity was 50 mg/kg/day.
| Purity | : | 99 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral(gavage) |
| Doses | : | 0(vehicle), 12.5, 50, 200 mg/kg/day |
| Number of animals/group | : | Males, 13; females, 13 |
| Vehicle | : | Water for injection |
| Administration period | : | Males, 42 days Females, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Males, day 43 of administration Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
The NOEL for systemic toxicity is considered to be 12.5 mg/kg/day in male and female rats, and that for reproductive and developmental toxicity 50 mg/kg/day.
| 1) | The test was performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |