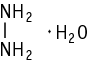
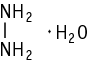
A repeated dose toxicity test was conducted at doses of 0, 1, 3, 10 and 30 mg/kg/day using male and female rats. Wasting, piloerection and salivation were observed in both sexes of the 30 mg/kg group. Dirty nasal discharge was observed in females of the 30 mg/kg group. Body weight gain, food consumption and food efficiency were suppressed in both sexes of the 30 mg/kg group.
Hematological examination revealed anemia in both sexes of the 30 mg/kg group and methemoglobinemia in both sexes in the 30 mg/kg group and in females of the 10 mg/kg group.
The serum levels of total bilirubin, A/G and inorganic phosphorus were elevated, while the glucose and chloride levels were reduced in both sexes of the 30 mg/kg group. Increase of the BUN and albumin values was noted in males and of calcium in females of the 30 mg/kg group.
The absolute and relative weights of the liver and kidneys were increased or tended to be elevated in both sexes of the 10 and 30 mg/kg groups. The absolute and relative spleen weights were increased in both sexes of the 30 mg/kg group.
Macroscopically, pale coloration of the liver was observed in both sexes of the 30 mg/kg group.
Histopathologically, fatty change of the hepatocytes was observed in males of the 10 and 30 mg/kg groups and in females of all groups, including the control group. Pigmentation of the spleen was observed in both sexes of the 30 mg/kg group. Extramedullary hematopoiesis was observed in both sexes of the 30 mg/kg group and in males of the 10 mg/kg group to a higher degree than in the controls.
The NOEL for repeat dose toxicity is considered to be 3 mg/kg/day for both sexes.
Hydrazine monohydrate was studied for repeated dose oral toxicity in rats in the OECD Test Guideline 421 at doses of 0, 2, 6 and 18 mg/kg.
Salivation was observed in both sexes in the 6 mg/kg and higher dose groups. In males of the 18 mg/kg group, suppression of body weight gain and decrease in food consumption were observed. On gross and histological examination, effects of the test substance on the liver and the spleen were observed in both sexes of the 6 and 18 mg/kg groups. Effects of the test substance on the kidney were noted in females of the 6 and 18 mg/kg groups and in males of the 18 mg/kg group.
The NOEL for repeated dose toxicity is considered to be 2 mg/kg/day for parental animals of both sexes.
In terms of reproductive/developmental toxicity, no adverse effects were observed in males of the 18 mg/kg group. Females of the 18 mg/kg group could not maintain pregnancy. In the 6 mg/kg group, a tendency for lowered body weights of pups and lowering of the viability index were observed on day 4 of lactation.
The NOELs for reproductive and developmental toxicity are considered to be 18 mg/kg/day for males, 6 mg/kg/day for females, and 2 mg/kg/day for offspring.
Hydrazine monohydrate proved mutagenic in Salmonella typhimurium TA100, TA1535, TA1537 and Escherichia coli WP2 uvrA/pKM101 without an exogenous metabolic activation system, and in Salmonella typhimurium TA100, TA1535 and Escherichia coli WP2 uvrA/pKM101 with an exogenous metabolic activation system.
Hydrazine monohydrate induced structural chromosomal aberrations in CHL/IU cells after short term treatment with and without an exogenous metabolic activation system. Polyploidy was not induced in any treatment group.
| Purity | : | 100.15 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 100, 130, 169, 220, 286 mg/kg/day |
| Number of animals | : | Males and females; 5 |
| Vehicle | : | Water for injection |
| GLP | : | Yes |
Test results:
| Purity | : | 100.15 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | Guideline for the 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 1, 3, 10, 30 mg/kg/day |
| Number of animals | Males and females; 5 | |
| Vehicle | : | Water for injection |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Males and females, days 29 or 43 |
| GLP | : | Yes |
Test results:
In the blood chemical examination, the serum levels of total bilirubin, A/G and inorganic phosphorus were elevated, while the glucose and chloride levels were lowered in both sexes of the 30 mg/kg group. Increase of the BUN and albumin values in males and of calcium in females of the 30 mg/kg group was noted.
The absolute weights of the kidneys were increased or tended to be increased in both sexes of the 10 and 30 mg/kg groups. Elevation of liver weights in females of the 10 and 30 mg/kg groups and of spleen weights in 30 mg/kg females was apparent. Relative weights of the liver and kidneys were higher or tended to be higher in both sexes of the 10 and 30 mg/kg groups than in controls. The relative spleen weights were increased in both sexes of the 30 mg/kg group.
Macroscopically, pale colored livers were observed in both sexes of the 30 mg/kg group. Histopathologically, fatty change of the hepatocytes was observed in males of the 10 and 30 mg/kg groups and in females of all groups, including the controls. Pigmentation in the spleen was observed in both sexes of the 30 mg/kg group. A higher degree of extramedullary hematopoiesis than normal was observed in both sexes of the 30 mg/kg group and in males of the 10 mg/kg group.
The NOEL for repeated dose toxicity is considered to be 3 mg/kg/day for both sexes.
| Purity | : | 100.15 % |
| Test species/strains | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 2, 6, 18 mg/kg/day |
| Number of animals/group | Males, 12; females, 12 | |
| Vehicle | : | Water for injection |
| Administration period | : | Males, 48 days Females, from 14 days before mating to day 3 of lactation |
| Terminal killing | : | Males, day 49 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
Two males of the 18 mg/kg group died. Salivation was observed in males and females of the 6 mg/kg and higher dose groups. Lacrimation was observed in females of the 18 mg/kg group. Decrease in body weight and lowered food consumption were observed in males of the 18 mg/kg group in the early stage of the administration period. In males of the 18 mg/kg group, suppression of body weight gain was also observed.
On gross and histological examination, relative liver weights and kidney weights were higher than normal in males of the 18 mg/kg group. Relative spleen weights and liver weights were elevated in females of the 6 mg/kg group. Pale liver as a gross finding, and fatty change of liver and pigmentation of spleen as histological findings were observed in males of the 6 mg/kg group and in males and females of the 18 mg/kg group. Moreover, effects of administration of the test substance on the heart were observed in males of the 18 mg/kg group.
The NOEL for repeated dose toxicity is considered to be 2 mg/kg/day for parental animals in both sexes.
<Reproductive and developmental toxicity>
The test substance had no effects on either copulation or fertility ability. In females of the 18 mg/kg group, lethal action on embryos was observed.
There was no abnormality considered to be the effect of the test substance on external observation of pups of the 6 mg/kg group but body weights and the viability index on Day 4 of lactation tended to be lower in males and females.
There were no gross abnormalities considered to be the effects of the test substance in dead fetuses or offspring necropsied.
The NOELs for reproductive and developmental toxicity are considered to be 18 mg/kg/day for males, 6 mg/kg/day for females, and 2 mg/kg/day for offspring.
| Purity | : | 100.15 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA/pKM101 |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Vehicle | : | Water for injection |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98), Sodium azide (TA1535), 9-Aminoacridine hydrochloride (TA1537) and N-Ethyl-N'-nitro-N-nitrosoguanidine (WP2 uvrA/pKM101) +S9 mix; 2-Aminoanthracene (five strains) |
| Dosage | : | -S9 mix; 0, 9.77, 19.5, 39.1, 78.1, 156, 313, 625 μg/plate (TA100) -S9 mix; 0, 4.88, 9.77, 19.5, 39.1, 78.1, 156 μg/plate (TA1535, WP2 uvrA/pKM101) -S9 mix; 0, 9.77, 19.5, 39.1, 78.1, 156, 313 μg/plate (TA98, TA1537) +S9 mix; 0, 39.1, 78.1, 156, 313, 625, 1250 μg/plate (TA100) +S9 mix; 0, 4.88, 9.77, 19.5, 39.1, 78.1, 156 μg/plate (TA1535) +S9 mix; 0, 1.22, 2.44, 4.88, 9.77, 19.5, 39.1, 78.1 μg/plate (WP2 uvrA/pKM101) +S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (TA98, TA1537) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] |
| With metabolic activation: | [*] | [ ] | [ ] |
Escherichia coli WP2 uvrA/pKM101
| + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] |
| With metabolic activation: | [*] | [ ] | [ ] |
| Purity | : | 100.15 % |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Vehicle | : | Isotonic sodium chloride solution |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Benzo[a]pyrene |
| Dosage | : | -S9 mix (6 hr short-term treatment); 0, 62.5, 125, 250, 500 μg/mL +S9 mix (6 hr short-term treatment); 0, 125, 250, 500 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| Lowest concentration producing cytogenetic effects in vitro | : | ||
| Without metabolic activation (short-term treatment) | : | 0.0625 mg/mL (clastogenicity) | |
| With metabolic activation (short-term treatment) | : | 0.25 mg/mL (clastogenicity) | |
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-Pyo Center), 582-2 Shiosinden Arahama, Fukude-Cho, Iwata-Gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |
| 2) | The tests were performed by the Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki 314-0255, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |