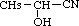
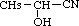
2-Hydroxypropanenitrile was studied for oral toxicity in rats in a single dose toxicity test at doses of 15, 21, 30, 42 and 60 mg/kg in males, and 21, 30, 42, 60 and 84 mg/kg in females and in an OECD combined repeat dose and reproductive/developmental toxicity screening test at doses of 0, 1.2, 6 and 30 mg/kg/day. 2-Hydroxypropanenitrile was also tested for mutagenicity with assays for reverse mutation in bacteria and chromosomal aberrations in cultured Chinese hamster (CHL) cells.
The single dose oral toxicity test revealed LD50s of 31.0 mg/kg in males and 41.1 mg/kg in females.
For repeat dose toxicity, a transient reduction of locomotor activity, as well as hypopnea and salivation were noted in the 30 mg/kg/day males and females, and macroscopically hypertrophy of the liver was evident in the 30 mg/kg males. Histopathologically, centrilobular hypertrophy and fatty change of hepatocytes were observed in the 30 mg/kg males. Blood chemical analyses showed a significant decrease in GOT, and significant increases in total protein, albumin and Ca in the 30 mg/kg males. The NOEL for repeat dose toxicity was 6 mg/kg/day.
2-Hydroxypropanenitrile was found to have no effects on any reproductive /developmental parameters. NOELs for reproductive performance and offspring development were both 30 mg/kg/day.
2-Hydroxypropanenitrile was not mutagenic for S. typhimurium TA100, TA1535, TA98, TA1537 and E. coli WP2 uvrA with or without exogenous metabolic activation up to 5000 μg/plate. However this chemical induced chromosomal aberrations with and without exogenous metabolic activation at concentrations of 0.71 and 0.38 mg/ml, respectively. Polyploidy was also weakly induced after 48 h treatment.
| Purity | : | 92.3% |
| Test species/strains | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Doses | : | Male: 15, 21, 30, 42, 60 mg/kg Female: 21, 30, 42, 60, 84 mg/kg |
| Number of animals | : | Male, 5; Female, 5 |
| GLP | : | Yes |
| Test results | : | LD50: Male, 31.0 mg/kg; Female, 41.1 mg/kg |
| Purity | : | 92.3% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 1.2, 6, 30 mg/kg/day |
| Number of animals | : | Male, 10; Female, 10 |
| Vehicle | : | Distilled water |
| Administration period | : | Male, 43days Female, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Male, day 43 Female, day 4 of lactation |
| GLP | : | Yes |
Test results:
Histopathologically, centrilobular hypertrophy (swelling) and fatty changes of hepatocytes were observed in males given 30 mg/kg. Blood chemical analyses for 30 mg/kg males revealed a significant decrease in GOT, and significant increase in total protein, albumin and Ca. All values obtained by hematological analysis were normal.
No changes were detected in body weight or food consumption in either sex.
<Reproductive and developmental toxicity>
The parental animals exhibited no effects on reproductive parameters including copulation index, fertility index, gestation period, behavior at delivery and lactation. There were no significant differences in offspring parameters including number of offspring, live offspring, sex ratio, survival index and body weight. The macroscopic and microscopic findings did not show any changes due to the test substance.
| Purity | : | 92% |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guideline for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedure | : | Plate method |
| Solvent | : | DMSO |
| Positive controls | : | -S9, AF-2 (TA100, WP2 uvrA, TA98), azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-Aminoanthracene (all strains) |
| Doses | : | TA100, TA1535, TA98, TA1537: 0, 4.69, 9.39, 18.75, 37.5, 75, 150 μg/plate WP2: 0, 75, 150, 300, 600, 1200, 2400 ug/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicate | : | 2 |
| GLP | : | Yes |
Genotoxic effects:
S.typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 92% |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | water |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9: 0, 0.10, 0.19, 0.38 mg/ml +S9: 0, 0.18, 0.36, 0.71 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| + | ? | - | |
| with metabolic activation: | [*] | [ ] | [ ] |
| without metabolic activation: | [*] | [ ] | [ ] |
| 1) | The tests were performed by the Mitsubishi-Kasei Institute of Toxicological and Environmental Sciences, 14 Sunayama, Hazaki-machi, Kashima-gun, Ibaraki, 314-02, Japan. Tel 81-479-46-2871 Fax 81-479-46-2874 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |