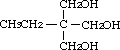

2-Ethyl-2-hydroxymethyl-1,3-propanediol was studied for oral toxicity in rats in an OECD combined repeat dose and reproductive/developmental toxicity screening test at doses of 0, 12.5, 50, 200 and 800 mg/kg/day, and for mutagenicity with assays for reverse mutation in bacteria and chromosomal aberrations in cultured Chinese hamster (CHL) cells.
For repeat dose toxicity, body weights of both males and females given 800 mg/kg were lower than those of controls. Absolute and relative liver weights were elevated significantly in male rats receiving 800 mg/kg. Although gross findings at necropsy revealed hypertrophy of the liver, histopathological examination showed no evidence of any related cytoplasmic changes. The NOEL for repeat dose toxicity was achieved at 200 mg/kg/day. No signs indicative of reproductive/developmental toxicity were observed. NOELs for reproductive performance and offspring development were both 800 mg/kg/day.
The mutagenicity tests were both negative. 2-Ethyl-2-hydroxymethyl-1,3-propanediol was not mutagenic for bacteria either with or without exogenous metabolic activation up to 5000 μg/plate. Neither chromosomal aberrations nor polyploidy were induced in CHL cells up to 1.34 mg/ml with or without exogenous metabolic activation.
| Purity | : | 99.9 % |
| Test species/strains | : | Rat/Slc:SD |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 12.5, 50, 200, 800 mg/kg/day |
| Number of animals | : | Male, 12; Female, 12/group |
| Vehicle | : | Distilled water |
| Administration period | : | Male, 45 days Female, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Male, day 46 Female, day 4 of lactation |
| GLP | : | Yes |
Test results:
There were no observed effects of the test substance on hematological findings of the treated male rats. Blood chemical examination revealed no changes indicating any specific organ dysfunctions. Absolute and relative liver weights were elevated significantly in male rats receiving 800 mg/kg and increased but not significantly, in female rats receiving 800 mg/kg.
Necropsy revealed hypertrophy of the liver in 3 male rats receiving 800 mg/kg. However, histopathological examination revealed no definite morphological lesions.
Slight basophilic alteration of the renal tubular epithelial cells was observed in 1 female rat receiving 50 mg/kg, in 2 female rats receiving 200 mg/kg and in 5 female rats receiving 800 mg/kg. However, these changes were not unequivocally attributable to the test substance administration, because of their limited distribution and limited degree, and because similar lesions were observed in male rats of all groups including the controls.
<Reproductive and developmental toxicity>
There were no effects of the test substance on copulation, fertility or estrus cycle of rats. Delivery was normal for all dams. No effects of the test substance were observed on dams during the lactation period. External examination of pups revealed no increase in the incidence of abnormalities. Body weight gain of pups was normal up to day 4 of the lactation period. Stillborn, dead pups and pups killed at day 4 of lactation period showed no abnormal gross lesions be attributable to treatment with the test substance.
| Purity | : | 99.9% |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Distilled water |
| Positive control | : | -S9, AF-2 (TA100, WP2 uvrA, TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-aminoanthracene (all strains) |
| Doses | : | 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Genotoxic effects:
S. typhimurium TA100, TA1535, TA98, TA1537, TA1538
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.9% |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Japanese Guidelines for Screening Mutagenicity Testing of Chemicals |
| Solvent | : | Distilled water |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9, 0, 0.34, 0.67, 1.34 mg/ml +S9, 0, 0.34, 0.67, 1.34 mg/ml |
| S-9 | : | Rat liver, induced by phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| 1) | The test was performed by the Biosafety Research Center/Foods, Drugs and Pesticides (An-pyo Center), 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka, 437-12, Japan. Tel. 81-538-58-1266 Fax 81-538-58-1393 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel. 81-463-82-4751 Fax 81-463-82-9627 |