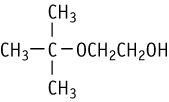

2-tert-Butoxyethanol was studied for oral toxicity in rats in an OECD combined repeated dose and reproductive/developmental toxicity screening test at doses of 0, 4, 20, and 100 mg/kg.
With regard to repeated dose toxicity, the following results were obtained. No deaths occurred in any of the treated groups. Hematological examination revealed decrease in erythrocyte count, hemoglobin and MCHC, and increase in MCV, MCH and reticulocytes in both sexes of the 100 mg/kg group. Similar changes were recognized in females of the 20 mg/kg group. In addition, decreases in hematocrit and leukocyte count were observed in males of the 100 mg/kg group. Decrease of MCHC was observed in males of the 20 mg/kg group. Histopathologically, the test substance had effects on the spleen, liver, kidneys and bone marrow in both sexes of the 20 and 100 mg/kg groups. These changes can be summarized as indicating an increase in hematogenous functions and hemosiderin deposition. Both sexes in the 100 mg/kg group showed chromaturia as a clinical sign, increase in spleen weights, and spleen enlargement. The NOEL for repeated dose toxicity is considered to be 4 mg/kg/day for both sexes.
With regard to reproductive and developmental toxicity, the compound had no effects on any relevant parameters. The NOEL for reproductive and developmental toxicity is considered to be 100 mg/kg/day for parental animals and offspring.
Reverse mutation assays using microorganisms (Salmonella typhimurium, Escherichia coli)were conducted to assess the potential of 2-tert-butoxyethanol to induce gene mutations.
2-tert-Butoxyethanol did not induce gene mutations in bacteria under the conditions of this study.
In vitro chromosomal aberration tests using cultured cells (CHL/IU) were conducted to assess the potential of 2-tert-butoxyethanol to induce chromosomal aberrations.
2-tert-Butoxyethanol did not induce chromosomal aberrations in cultured cells under the conditions of this study.
| Purity | : | 99.98 % |
| Test species/strain | : | Rats/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 500, 1000, 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Purified water |
| GLP | : | Yes |
Test results:
The LD50 value is considered to be more than 2000 mg/kg for both sexes.
| Purity | : | 99.98 % |
| Test species/strain | : | Rats/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 4, 20, 100 mg/kg |
| Number of animals/group | Males, 12; females, 12 | |
| Vehicle | : | Purified water |
| Administration period | : | Males, 36 days Females, from 14 days before mating to day 4 of lactation |
| Terminal killing | : | Males, day 37 Females, day 5 of lactation |
| GLP | : | Yes |
Test results:
Hematological examination revealed decreases in erythrocyte count, hemoglobin and MCHC, and increases in MCV, MCH and reticulocytes in both sexes of the 100 mg/kg group. Similar changes were also recognized in females of the 20 mg/kg group. In addition, decreases in hematocrit and leukocytes count were observed in males of the 100 mg/kg group and decrease of MCHC was noted in males of the 20 mg/kg group. Histopathologically, the test substance had effects on spleen, liver, kidney and bone marrow in both sexes of the 20 and 100 mg/kg groups. These changes can be summarized as indicating an increase in hematogenous function and hemosiderin deposition. Both sexes of the 100 mg/kg group showed chromaturia as a clinical sign, as well as increase in spleen weights and spleen enlargement.
The NOEL for repeated dose toxicity is considered to be 4 mg/kg/day for both sexes.
<Reproductive and developmental toxicity>
The compound had no effects on reproductive parameters such as the mating index, the fertility index, numbers of corpora lutea or implantations, the implantation index, the delivery index, the gestation index, gestation length, parturition or maternal behavior. On examination of neonates, there was no significant variation in numbers of offspring or live offspring, the sex ratio, the live birth index, the viability index or body weight. No abnormal findings ascribable to the compound were found for external features or clinical signs or at necropsy of the offspring.
The NOEL for reproductive and developmental toxicity is considered to be 100 mg/kg/day for parental animals and offspring.
| Purity | : | 99.98 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals(Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Water for injection |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98 and WP2 uvrA), Sodium azide (TA1535), 9-Aminoacridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate (all strains for dose-finding study) +S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate (all strains for dose-finding study) -S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (all strains for main study) +S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (all strains for main study) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535,TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.98 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals(Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Water for injection |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 300, 600, 1200 μg/mL +S9 mix (short-term treatment); 0, 300, 600, 1200 μg/mL -S9 mix (continuous treatment 24 hr); 0, 300, 600, 1200 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Kashima Laboratory, Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki, 314-0255, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), 582-2 Shioshinden, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |