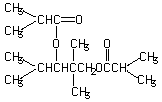
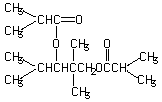
With regard to repeat dose toxicity, inhibition of body weight gain was observed in males given 750 mg/kg. Food consumption increased in females given 750 mg/kg. Blood chemical examination revealed increases in levels of creatinine and total bilirubin in males given 150 and 750 mg/kg, and an increase in total protein levels in males given 750 mg/kg. Liver weights were elevated significantly in the 150 and 750 mg/kg dose males and in the 750 mg/kg dose females. Moreover, kidney weights were elevated significantly in the 750 mg/kg dose males. Necropsy revealed brown colored livers in males given 750 mg/kg. Histopathological examination showed increases in the grade of basophilic change of renal tubular epithelium and hyaline droplet degeneration in males given 150 and 750 mg/kg. Moreover, necrosis and fibrosis of the proximal tubules, dilatation of the distal tubules, decreased fatty change and swelling of the liver cells were seen in males given 750 mg/kg. NOELs for repeat dose toxicity are considered to be 30 mg/kg/day in males and 150 mg/kg/day in females. In terms of reproductive/developmental toxicity, the compound showed no effects on any relevant parameters. NOELs for reproductive performance in males and females and for pups development are considered to be both 750 mg/kg/day.
2,2,4-Trimethyl-1,3-pentanediol diisobutyrate was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA. Neither structural nor numerical chromosomal aberrations were induced in CHL/IU cells up to the concentration going 50% cell growth inhibition or the limit concentration of 10 mM, in the absence or presence of an exogenous metabolic activation system.
| Purity | : | 99.7% |
| Test species/strain | : | Rats/Slc:SD |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral(gavage) |
| Doses | : | 0(Vehicle),30,150,750mg/kg/day |
| Number of animals | : | Male, 12; female, 12/group |
| Vehicle | : | Corn oil |
| Administration period | : | Male, 44 days
Female,from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Male, day 45 Female,day 4 of lactation |
| GLP | : | Yes |
Test results:
There were no observed effects of the test substance on hematological findings for the treated males. Blood chemical examination revealed increases in levels of creatinine and total bilirubin in males given 150 and 750 mg/kg, and an increase in the total protein level in the 750 mg/kg dose males. Relative liver weights were elevated significantly in the 150 and 750 mg/kg dose males. Moreover, the absolute liver weight and absolute and relative kidney weights were also elevated significantly in the 750 mg/kg dose males. Absolute and relative liver weights were elevated similarly significantly in the 750 mg/kg dose females. Necropsy revealed brown colored livers in males given 750 mg/kg. Histopathological examination showed increases in the grade of basophilic change of renal tubular epithelium and hyaline droplet degeneration in males given 150 and 750 mg/kg. Moreover, necrosis and fibrosis of the proximal tubules, dilatation of the distal tubules, decreased fatty change and swelling of the liver cells were seen in males given 750 mg/kg.
NOELs for repeat dose toxicity are considered to be 30 mg/kg/day for males and 150 mg/kg/day for females.
NOELs for reproductive performance of males and females and for pups development are considered to be both 750 mg/kg/day.
| Purity | : | 99.7% |
| Test species/strains | : | S.typhimurium TA100, TA1535, TA98, TA1537, E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Plate incorporation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9, AF-2 (TA100, WP2, TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-aminoanthracene (all strains) |
| Dosage | : | 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
| + | ? | - | |
| with metabolic activation | [ ] | [ ] | [*] |
| without metabolic activation | [ ] | [ ] | [*] |
| with metabolic activation | [ ] | [ ] | [*] |
| without metabolic activation | [ ] | [ ] | [*] |
| Purity | : | 99.7% |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9 (continuous treatment): 0, 0.01, 0.02, 0.04 mg/ml
-S9 (short-term treatment): 0, 0.005, 0.009, 0.018 mg/ml +S9 (short-term treatment): 0, 0.33, 0.65, 1.30 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The test was performed by the Biosafety Research Center, Foods, Drugs, and Pesticides (An-pyo Center), 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka 437-12, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |