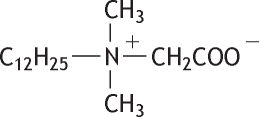
Molecular formula: C16H33NO2 Molecular weight: 271.44
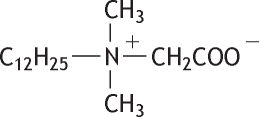
N-(Carboxymethyl)-N,N-dimethyl-1-dodecanaminium, inner salt, was studied for oral toxicity in female rats according to OECD Test Guideline 423. Doses were set at 300 mg/kg (1st and 2nd steps) and 2000 mg/kg (3rd step).
No dead animals were observed in the 300 mg/kg group, and there were no changes in clinical signs, body weights, or necropsy findings. All animals receiving 2000 mg/kg died within 2 days of administration. Decrease in locomotor activity, irregular respiration, loose stool, and soiled perineal regions were noted before death. At necropsy, effects on the digestive system were noted.
From the above results, the test substance was classified into category 4(>300-2000 mg/kg) of the GHS(Globally Harmonized Classification System).
N-(Carboxymethyl)-N,N-dimethyl-1-dodecanaminium, inner salt, was studied for oral toxicity in rats in an OECD combined repeated dose and reproductive/developmental toxicity screening test at doses of 0, 10, 60, and 300 mg/kg/day.
Regarding repeated dose toxicity, in the 300 mg/kg group, 2 females died on days 20 and 23 of gestation, and salivation was noted in all animals of both sexes. As necropsy findings, there were thickening, yellowish change and/or raising of the forestomach mucosa. Correspondingly to these findings, histopathological changes included squamous cell hyperplasia, hyperkeratosis, parakeratosis, inflammatory cell infiltration, erosion and edema in the forestomach and/or glandular stomach of both sexes. Other histopathological findings, degeneration/necrosis of the tubular epithelium and hyperplasia of the pelvic epithelium in the kidneys, and hyperplasia of the mucosal epithelium in the urinary bladder, were observed in the 60 and 300 mg/kg groups of both sexes. Blood chemistry examination revealed increases in urea nitrogen in the 300 mg/kg group of both sexes. The changes demonstrated recovery or a tendency for reversibility in the recovery and/or satellite groups of both sexes. The NOAEL for repeated dose toxicity is considered to be 10 mg/kg/day for both sexes.
As to reproductive and developmental toxicity, in the 300 mg/kg group, total litter loss was noted in one dam on day 0 of lactation, and extension of the gestation length, decreases in total numbers of offspring, and low gestation index were noted. The NOAELs for reproductive and developmental toxicity are considered to be 300 mg/kg/day for males and 60 mg/kg/day for females and offspring.
N-(Carboxymethyl)-N,N-dimethyl-1-dodecanaminium, inner salt, was not found to be mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA/pKM101, with or without an exogenous metabolic activation system.
N-(Carboxymethyl)-N,N-dimethyl-1-dodecanaminium, inner salt, did not induce structural chromosomal aberrations or polyploidy in CHL/IU cells, with or without exogenous metabolic activation.
| Purity | : | 27.1 % |
| Test species/strain | : | Rats/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 423 |
| Route | : | Oral(gavage) |
| Dosage | : | 300 mg/kg (1st and 2nd steps) and 2000 mg/kg (3rd step) |
| Number of animals/group | : | Females, 6(300 mg/kg), 3(2000 mg/kg) |
| Vehicle | : | Water for injection |
| GLP | : | Yes |
Test results:
| Purity | : | 27.1 % |
| Test species/strain | : | Rats/Crj: CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral(gavage) |
| Dosage | : | 0(vehicle), 10, 60, 300 mg/kg |
| Number of animals/group | : | Males, 12; females, 12 Satellite females, 5(0 and 300 mg/kg groups) |
| Vehicle | : | Water for injection |
| Administration period | : |
Males and satellite females, 42 days |
| Terminal killing | : |
Males, day 43 and day 57 |
| GLP | : | Yes |
Test results:
N-(Carboxymethyl)-N,N-dimethyl-1-dodecanaminium, inner salt, was studied for oral toxicity in rats in an OECD combined repeated dose and reproductive/developmental toxicity screening test at doses of 0, 10, 60 and 300 mg/kg/day.
In the 300 mg/kg group, deaths were observed of one female each on days 20 and 23 of gestation. Salivation was noted in all animals of both sexes. As necropsy findings, there were thickening, yellowish change and/or raising of forestomach mucosa. Corresponding to these findings, histopathological changes included squamous cell hyperplasia, hyperkeratosis, parakeratosis, inflammatory cell infiltration, erosion and edema in the forestomach and/or glandular stomach of the both sexes.Other histopathological findings, degeneration/necrosis of the tubular epithelium, hyperplasia of the pelvic epithelium in the kidneys, and hyperplasia of the mucosal epithelium in the urinary bladder were observed in the 60 and 300 mg/kg groups of both sexes. Blood chemistry examination revealed increases in urea nitrogen in the 300 mg/kg group of both sexes. The above changes were apparent at the end of the dosing period; however, they had disappeared within the 2-week recovery period, or had become less pronounced concerning their incidence and degree, and thus were confirmed to be reversible.
From these findings, the NOAEL for repeated dose toxicity is considered to be 10 mg/kg/day for both sexes.
<Reproductive and developmental toxicity>
In the 300 mg/kg group, total litter loss was noted in one dam on day 0
of lactation. This group also demonstrated increase in the gestation length
and decrease in the total number of offspring. The compound had no effects
on other reproductive parameters such as the estrous cycle, mating index,
fertility index, number of corpora lutea or implantations, implantation
index, delivery index, parturition or maternal behavior. On examination
of neonates, a low gestation index accompanied by decrease in the total
number of offspring in the 300 mg/kg group were observed. No effects were
noted on other neonatel parameters such as the sex ratio, viability index,
external features, clinical signs, body weight, or necropsy findings.
The NOAELs for reproductive and
developmental toxicity are considered to be 300 mg/kg/day for males and 60
mg/kg/day for females and offspring.
| Purity | : | 27.1 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA/pKM101 |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Vehicle | : | Water for injection |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, WP2 uvrA/pKM101, TA98), sodium azide (TA1535) and 9-aminoacridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 2.44, 4.88, 9.77, 19.5, 39.1, 78.1 μg/plate (TA100, TA1535), 0, 2.44, 4.88, 9.77, 19.5, 39.1, 78.1, 156 μg/plate (WP2 uvrA/pKM101), 0, 0.610, 1.22, 2.44, 4.88, 9.77, 19.5, 39.1 μg/plate (TA98, TA1537) +S9 mix; 0, 9.77, 19.5, 39.1, 78.1, 156, 313 μg/plate (TA100, TA1535), 0, 39.1, 78.1, 156, 313, 625, 1250, 2500 μg/plate (WP2 uvrA/pKM101), 0, 9.77, 19.5, 39.1, 78.1, 156, 313, 625 μg/plate (TA98, TA1537) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
This chemical did not induce gene mutations in the Salmonella typhimurium and Escherichia coli strains. Toxicity was observed at 39.1 μg/plate or more (TA100, TA1535), 19.5 μg/plate or more (TA98, TA1537) and 78.1 μg/plate or more (WP2 uvrA/pKM101) without metabolic activation, and at 313 μg/plate (TA100, TA1535), 313 μg/plate or more (TA98, TA1537) and 1250 μg/plate or more (WP2 uvrA/pKM101) with metabolic activation.
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 27.1 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Vehicle | : | Isotonic sodium chloride solution |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Benzo [a] pyrene |
| Dosage | : | -S9 mix (6 hr short-term treatment); 100, 125, 150, 175, 200 μg/mL +S9 mix (6 hr short-term treatment); 200, 225, 250, 275, 300 μg/mL -S9 mix (24 hr continuous treatment; main test); 50, 75, 100, 125, 150 μg/mL -S9 mix (24 hr continuous treatment; additional test); 100, 110, 120, 130, 140, 150 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
This chemical did not induce structural chromosomal aberrations or polyploidy under the conditions of this experiment.
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Kashima Laboratory, Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Kamisu-shi, Ibaraki, 314-0255, Japan. Tel +81-479-46-2871, Fax +81-479-46-2874 |