n-Pentadecane
n-ペンタデカン
CAS No. 629-62-9
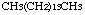
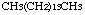
n-Pentadecane was studied for oral toxicity in rats in a 28-day repeat dose toxicity test at doses of 0, 100, 300 and 1000 mg/kg/day and for mutagenicity with assays for reverse mutation in bacteria and chromosomal aberrations in cultured Chinese hamster (CHL) cells.
n-Pentadecane did not show any toxic effects in the repeat dose study. NOELs for repeat dose toxicity were 1000 mg/kg/day for both sexes.
n-Pentadecane was not mutagenic to S. typhimurium TA100, TA1535, TA98, TA1537 and E. coli WP2 uvrA with or without exogenous metabolic activation up to 5000 μg/plate. This chemical induced neither chromosomal aberrations nor polyploidy in CHL cells with or without exogenous metabolic activation up to 1.5 mg/ml.
| Purity | : | 99 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 100, 300, 1000 mg/kg/day |
| Number of animals | : | Male, 5; Female, 5 |
| Vehicle | : | 0.5 % CMC Na solution with 0.1% Tween 80 |
| Administration period | : | Male and Female, 28 days |
| Terminal kill | : | Male and Female, days 29 or 43 |
| GLP | : | Yes |
Test results:
There were no changes in organ weight. No abnormalities were seen that could be attributed to treatment with the test substance on macroscopic or microscopic pathological examinations.
| Purity | : | > 99% |
| Test species/strain | : | S. typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedure | : | Plate method |
| Solvent | : | Acetone |
| Positive control | : | -S9, AF-2 (TA100,WP2 uvrA,TA98), sodium azide (TA1525) and 9-aminoacridine (TA1537) +S9; 2-Aminoanthracene (all strains) |
| Doses | : | 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicate | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
S. typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99 % |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9, 0, 0.37, 0.75, 1.5 mg/ml +S9, 0, 0.37, 0.75, 1.5 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Lowest concentration producing cytogenetic effects in vitro:
with metabolic activation: > 1.5 mg/ml
without metabolic activation: > 1.5 mg/ml
Genotoxic effects:
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| 1) | The test was performed by the Biosafety Research Center, Foods, Drugs and Pesticide (An-pyo Center), 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka, 437-12, Japan. Tel 81-538-58-1266 Fax 81-538-58-1393 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |