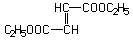
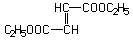
Diethyl fumarate was studied for oral toxicity in rats in a single dose toxicity test at doses of 910, 1180, 1540, 2000, 2600 and 3380 mg/kg, and in an OECD repeat dose and reproductive/developmental toxicity screening test at doses of 11, 30 and 100 mg/kg/day. Genotoxicity of diethyl fumarate was also studied by the reverse mutation assay in bacteria and the chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells.
The single dose toxicity test revealed LD50 values between 1540 and 2000 mg/kg for males and of 1367.45 mg/kg for females.
In the repeat dose toxicity test, histopathological examination of the forestomach revealed thickening of the mucosal layer in both sexes of all treated groups, and hyperkeratosis in males of all treated groups and in females receiving 30 and 100 mg/kg. These changes were dose-dependent. Edema in the submucosal tissue, ulcer and focal edema in the lamina propria mucosae and vesiculation in the superficial zone of mucosal layer were noted in the 30 or 100 mg/kg group. Absolute or relative organ weights of the kidney and liver increased in both sexes of the 100 mg/kg group. The NOEL for repeat dose toxicity in both sexes is considered to be less than 11 mg/kg/day. No effects were observed on reproductive performance in either sex and on development of the next generation. NOELs for reproductive and developmental performances are considered to be 100 mg/kg/day.
Diethyl fumarate was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA. This chemical substance induced structural chromosomal aberrations in CHL/IU cells without metabolic activation, but the response was diminished in the presence of an exogenous metabolic activation system. Numerical aberrations were not induced.
| Purity | : | 98% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Doses | : | 910, 1180, 1540, 2000, 2600, 3380 mg/kg |
| Number of animals | : | Male, 5; female, 5/group |
| GLP | : | Yes |
| Purity | : | 98% |
| Test species/strains | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (control), 11, 30, 100 mg/kg/day |
| Number of animals | : | Male, 12; female, 12/group |
| Control substance | : | Purified water |
| Administration period | : | Male, 46 days Female, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Male, day 47
Female, day 4 of lactation |
| GLP | : | Yes |
Test results:
The NOEL for repeat dose toxicity in both sexes is considered to be less than 11 mg/kg/day.
NOELs for reproductive and developmental performances are considered to be 100 mg/kg/day.
| Purity | : | 93.3% |
| Test species/strains | : | S.typhimurium TA100, TA1535, TA98, TA1537, E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Plate incorporation method |
| Solvent | : | Acetone |
| Positive controls | : | -S9, AF-2 (TA100, WP2, TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-aminoanthracene (all strains) |
| Dosage | : | without metabolic activation
0, 9.375, 18.75, 37.5, 75, 150, 300 μg/plate (TA100), 0, 78.13, 156.3, 312.5, 625, 1250, 2500μg/plate (TA1535, TA98, TA1537), 0, 156.3 312.5, 625, 1250, 2500, 5000 μg/plate (WP2) metabolic activation method 0, 312.5, 625, 1250, 2500 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
| + | ? | - | |
| with metabolic activation | [ ] | [ ] | [*] |
| without metabolic activation | [ ] | [ ] | [*] |
| with metabolic activation | [ ] | [ ] | [*] |
| without metabolic activation | [ ] | [ ] | [*] |
| Purity | : | 93.3% |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | DMSO |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9 (continuous treatment): 0, 0.003, 0.007, 0.013 mg/ml
-S9 (short-term treatment): 0, 0.008, 0.015, 0.030 mg/ml +S9 (short-term treatment): 0, 0.021, 0.042, 0.083 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| without metabolic activation (continuous treatment ) | : | 0.013 mg/ml (clastogenicity) 0.007 mg/ml (polyploidy) |
| without metabolic activation (short-term treatment) | : | 0.008 mg/ml (clastogenicity) 0.008 mg/ml (polyploidy) |
| with metabolic activation (short-term treatment) | : | > 0.083 mg/ml |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [*] | [ ] | [ ] | [*] | [ ] | [ ] |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Safety Research Institute for Chemical Compound Co., Ltd., 363-24, Shin-ei, Toyohira-ku, Sapporo, Hokkaido 004, Japan. Tel +81-11-885-5031 Fax +81-11-885-5313 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |