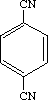
1,4-Dicyanobenzene
1,4-ジシアノベンゼン
CAS No. 623-26-7
Terephthalonitrile
テレフタロニトリル
Molecular formula: C8H4N2 Molecular weight: 128.14

The single dose toxicity test revealed an LD50 value of approximate 2000 mg/kg for both sexes.
In the repeat dose test, 1,4-dicyanobenzene caused hepatic changes such as increased relative organ weights in both sexes receiving 80 mg/kg group, and hypertrophy and increased smooth endoplasmic reticulum of centrilobular hepatocytes in males of the 80 mg/kg group, thyroidal changes such as increased relative organ weights, irregular shaped follicles and decreased colloid in males of the 20 mg/kg and above groups; renal changes such as pale discoloration and hyaline droplet deposition in the tubular epithelium in males of the 5 mg/kg and above groups and changes in urinary or serum electrolytes in both sexes receiving 20 mg/kg and more. Many of these findings disappeared by day 14 after withdrawal. The NOELs for repeat dose toxicity for males and for females are considered to be 1.25 mg/kg/day and 5 mg/kg/day, respectively.
1,4-Dicyanobenzene was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA.
Neither structural chromosomal aberrations nor polyploidy were induced in CHL/IU cells up to the limit concentration of 10 mM, in the absence or presence of an exogenous metabolic activation system.
| Purity | : |  99% 99% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | CD Test Guideline 401 |
| Dosage | : | 0, 354, 500, 707, 1000, 1414, 2000 mg/kg |
| Number of animals | : | Males, 5; females, 5/group |
| GLP | : | Yes |
Test results:
LD50: Male, ca. 2000 mg/kg; female, ca. 2000 mg/kg
| Purity | : |  99% 99% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Testing for Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 1.25, 5, 20, 80 mg/kg/day |
| Number of animals | : | Males, 7 and 14 (0, 80 mg/kg) Females, 7 and 14 (0, 80 mg/kg) |
| Vehicle | : | 0.5% MC solution |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Days 29 or 43 |
| GLP | : | Yes |
Test results:
| Purity | : | above 99% |
| Test species/strains | : | S.typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test methods | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Plate incorporation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 Mix, AF-2 (TA100, WP2, TA98) sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9 Mix, 2-aminoanthracene (all strains) |
| Doses | : | 0, 312.5, 625, 1250, 2500 and 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
| + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| without metabolic activation: | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] |
| Purity | : |  99% 99% |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | 0.5% Carboxymethylcellulose sodium solution |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9 (continuous treatment): 0, 0.3, 0.7, 1.3 mg/ml -S9 (short-term treatment): 0, 0.3, 0.7, 1.3 mg/ml +S9 (short-term treatment): 0, 0.3, 0.7, 1.3 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
| clastogenicity | polyploidy | ||||||
| + | ? | - | + | ? | - | ||
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] | |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] | |
| 1) | The tests were performed by the Safety Research Institute for Chemical Compounds Co., Ltd., 363-24, Shin-ei, Toyohira-ku, Sapporo, Hokkaido, 004, Japan. Tel +81-11-885-5031 Fax +81-11-885-5313 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |