
The single dose oral toxicity test revealed an LD50 value of more than 2000 mg/kg for both sexes.
With regard to repeated dose toxicity, no clinical signs were observed in males and females during the administration period. Blood biochemical examination revealed a decrease in chloride in both sexes, and increases in the A/G ratio and inorganic phosphorous in males of the 1000 mg/kg group. There were increases in relative kidney weights in males of the 250 mg/kg group and relative kidney and liver weights in both sexes of the 1000 mg/kg group. These changes showed a tendency to recover or had complete recovery by the end of 14-day recovery period. The NOELs are considered to be 60 mg/kg/day for males and 250 mg/kg/day for females.
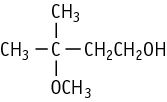
3-Methoxy-3-methyl-1-butanol was studied for oral toxicity in rats of both sexes in an OECD preliminary reproductive toxicity screening test at doses of 0, 8, 40, 200 and 1000 mg/kg.
There were increases of absolute and relative weights of the kidney in males of the 200 and 1000 mg/kg groups, and of the relative kidney and liver weights in females of the 1000 mg/kg group. The NOELs for repeated dose toxicity are considered to be 40 mg/kg/day for males and 200 mg/kg/day for females. The test substance showed no effects on any reproductive parameters of the parental animals or developmental parameters of the offspring. The NOELs for reproductive/developmetal toxicity are considered to be 1000 mg/kg/day for both reproductive performance of parental animals and offspring development.
Genotoxicity of 3-methoxy-3-methyl-1-butanol was studied by a reverse mutation test in bacteria. This substance was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 or Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
Genotoxicity of 3-methoxy-3-methyl-1-butanol was studied by a chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells.
3-Methoxy-3-methyl-1-butanol did not induce structural chromosomal aberrations or polyploidy up to 1.2 mg/mL (10 mmol/L) under the present test conditions.
| Purity | : | 99.19 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 1000, 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Distilled water |
| GLP | : | Yes |
Test results:
The LD50 value was estimated to be more than 2000 mg/kg for both sexes.
| Purity | : | 99.19 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | Guideline for the 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 15, 60, 250, 1000 mg/kg |
| Number of animals/group | Males, 5; females, 5 | |
| Vehicle | : | Distilled water |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Males and females, days 29 or 43 |
| GLP | : | Yes |
Test results:
| Purity | : | 99.19 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 8, 40, 200, 1000 mg/kg/day |
| Number of animals/group | Males, 12; females, 12 | |
| Vehicle | : | Distilled water |
| Administration period | : | Males, 47 days Females, from 14 days before mating to day 4 of lactation |
| Terminal killing | : | Males, day 48 Females, day 5 of lactation |
| GLP | : | Yes |
Test results:
Increases of absolute and relative weights of the kidney in males of the 200 and 1000 mg/kg groups were observed along with increases of the relative kidney and liver weights in females of the 1000 mg/kg group.
The NOELs for repeated dose toxicity are considered to be 40 mg/kg/day for males and 200 mg/kg/day for females.
<Reproductive and developmental toxicity>
The parental animals exhibited no alterations in reproductive parameters. There were also no significant differences in offspring parameters.
The NOELs for reproductive/developmental toxicity are considered to be 1000 mg/kg/day for reproductive performance of parental animals and for offspring development.
| Purity | : | 99.19 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Vehicle | : | Water for injection |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA), Sodium azide (TA1535) and 9-Aminoacridine (TA1537) +S9 mix; 2-Aminoanthracene (five strains) |
| Dosage | : | -S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (five strains) +S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (five strains) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 (1 for cytotoxicity test) |
| Number of replicates | : | 2 (plus 1 cytotoxicity test) |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA98, TA1535, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.19 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Vehicle | : | Water for injection |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (continuous treatment); 0, 0.30, 0.60, 1.2 mg/mL -S9 mix (short-term treatment); 0, 0.30, 0.60, 1.2 mg/mL +S9 mix (short-term treatment); 0, 0.30, 0.60, 1.2 mg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Research Institute for Animal Science in Biochemistry and Toxicology, 3-7-11 Hashimotodai, Sagamihara-shi, Kanagawa 229-1132, Japan. Tel +81-42-762-2775 Fax +81-42-762-7979 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |