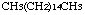
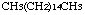
n-Hexadecane was studied for oral toxicity in single dose toxicity test at 500, 1000 and 2000 mg/kg, and in a 28-day repeat dose toxicity test at doses of 0, 8, 40, 200 and 1000 mg/kg/day. n-Hexadecane was also tested for mutagenicity with assays for reverse mutation in bacteria and chromosomal aberrations in cultured Chinese hamster (CHL) cells.
The single dose oral toxicity test revealed LD50s of above 2000 mg/kg in both sexes.
In the repeat dose study, hematological analyses showed a prolongation of prothrombin time in males of the 1000 mg/kg group, and a decrease in erythrocyte counts in females of the 200 and 1000 mg/kg groups. A significant increase in GPT was detected in males and females of the 1000 mg/kg group. These changes disappeared after a recovery period. NOELs for repeat dose toxicity were 200 mg/kg/day in males and 40 mg/kg/day in females.
n-Hexadecane was not mutagenic for S.typhimurium TA100, TA1535, TA98, TA1537, and E. coli WP2 uvrA with or without exogenous metabolic activation up to 5000 μg/plate. In addition, this chemical induced neither chromosomal aberrations nor polyploidy with or without an exogenous metabolic activation system up to 1 mg/ml.
| Purity | : | 99.8 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Doses | : | 500, 1000, 2000 mg/kg |
| Number of animals | : | Male, 5; Female, 5/group |
| GLP | : | Yes |
| Test results | : | LD50: Male > 2000 mg/kg, Female > 2000 mg/kg |
| Purity | : | 99.9 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 8, 40, 200, 1000mg/kg/day |
| Number of animals | : | Male, 10 or 15 (0, 1000 mg/kg); Female, 10 or 15 ( 0, 1000 mg/kg) |
| Vehicle | : | Corn oil |
| Administration period | : | Male, Female; 28 days |
| Terminal kill | : | Male, Female; days 29 or 43 |
| GLP | : | Yes |
Test results:
On hematological analyses, the 1000 mg/kg males showed significantly prolonged prothrombin time, 200 mg/kg and 1000 mg/kg females exhibited significant decrease in erythrocyte counts, and 1000 mg/kg females showed increased mean corpuscular hemoglobin. These changes disappeared after the recovery period. On blood biochemical analysis, a significant increase in GPT was detected in the1000 mg/kg males and females, but a return to normal value was evident after the recovery period.
No significant differences were noted in organ weights and pathological findings between any dose groups.
| Purity | : | 99.8% |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedure | : | Plate method |
| Solvent | : | Acetone |
| Positive controls | : | -S9, AF-2 (TA100,WP2 uvrA,TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-Aminoanthracene (all strains) |
| Doses | : | 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
S. typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.8 % |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9: 0, 0.25, 0.5, 1mg/ml +S9: 0, 0.25, 0.5, 1 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Lowest concentration producing cytogenetic toxicity:
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| 1) | The tests were performed by Nihon Bioresearch Inc., Hashima Laboratory, 6-104 Majima, Fukuju-cho, Hashima, Gifu, 501-62, Japan. Tel 81-583-92-6222 Fax 81-583-92-1284 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |