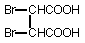
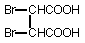
The single dose oral toxicity test revealed an LD50 of above 2000 mg/kg for both sexes.
In the repeat dose study, slight increased salivation was observed in males and females of the 1000 mg/kg/day group, but the frequency was only occasional at one or two times during the administration period. No test substance-related changes were noted in body weights, food consumption, and the findings obtained from hematology tests, blood chemical examination, urinalysis and pathological examination. The NOEL for the repeat dose toxicity is considered to be over 1000 mg/kg/day for both sexes.
2,3-Dibromosuccinic acid was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA. Neither structural nor numerical chromosomal aberrations were induced in CHL/IU cells up to the concentration going 50% cell growth inhibition or the limit concentration of 10 mM, in the absence or presence of an exogenous metabolic activation system.
| Purity | : | 99.8% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Dosage | : | 2000 mg/kg |
| Number of animals | : | Male, 5; female, 5/group |
| Vehicle | : | 0.5% Sodium Carboxymethyl Cellulose solution |
| GLP | : | Yes |
| Purity | : | 99.8% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Testing for Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 20, 140, 1000 mg/kg/day |
| Number of animals | : | Male, 6; female, 6/group |
| Vehicle | : | 0.5% Sodium Carboxymethyl Cellulose solution |
| Administration period | : | Male and female, 28 days |
| Terminal kill | : | Days 29 or 43 |
| GLP | : | Yes |
The NOEL for the repeat dose toxicity is considered to be over 1000 mg/kg/day for both sexes.
| Purity | : | > 98% |
| Test species/strains | : | S.typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Plate incorporation method |
| Solvent | : | Acetone |
| Positive controls | : | -S9, AF-2 (TA100, WP2, TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-aminoanthracene (all strains) |
| Dosage | : | without metabolic activation 0, 156.3, 312.5, 625, 1250, 2500,5000 μg/plate metabolic activation method 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Genetic effects:
S. typhimurium TA100, TA1535, TA 98, TA1537
| + | ? | - | |
| with metabolic activation | [ ] | [ ] | [*] |
| without metabolic activation | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| with metabolic activation | [ ] | [ ] | [*] |
| without metabolic activation | [ ] | [ ] | [*] |
| Purity | : | ≧ 98% |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Dosage | : | -S9 (continuous treatment): 0, 0.3, 0.7, 1.3 mg/ml -S9 (short-term treatment): 0, 0.7, 1.4, 2.8 mg/ml +S9 (short-term treatment): 0, 0.7, 1.4, 2.8 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Mitsubishi-Kasei Institute of Toxicological and Environmental Sciences (New name: Mitsubishi Chemical Safety Institute Ltd.), 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki 314-02, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |