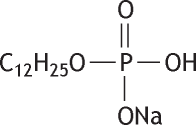
Molecular formula: C12H26O4NaP Molecular weight: 288.30

A single dose oral toxicity test of phosphoric acid, dodecyl ester, sodium salt in rats resulted in classification of the chemical into category 5 of the GHS regarding acute toxicity. There were no abnormalities in general condition, body weight or gross pathological findings in any animal.
Oral toxicity was studied in rats according to the OECD Test Guideline 422 at doses of 0, 250, 500 and 1000 mg/kg.
In the repeated dose oral toxicity test, gross pathological examination revealed recessed areas in the forestomach at 250 mg/kg and above and rough mucosa or white foci in the forestomach at 500 mg/kg and above, and erosion/ulceration, mucosal thickening and submucosal edema were apparent at 250 mg/kg and above on histopathological examination.
An additional test was conducted in order to determine the NOAEL with repeated administration, where the test article was administered to male and female rats at 62.5 or 125 mg/kg. In the additional test, submucosal edema in the forestomach was observed in females at 125 mg/kg.
The NOAEL for repeated dose oral toxicity test is judged to be 125 mg/kg/day for males and 62.5 mg/kg/day for females.
In items of reproductive/developmental toxicity, the test substance showed no adverse effects on any relevant parameters.
The NOAEL for the reproductive/developmental toxicity test is judged to be 1000 mg/kg/day for both male and female parent animals and offspring.
Reverse mutation assays using microorganisms (Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA) were conducted to assess the potential of phosphoric acid, dodecyl ester, sodium salt to induce gene mutations. No mutagenic activity was found in the bacteria under the present experimental conditions.
In vitro chromosomal aberration tests using cultured mammalian cells (CHL/IU) were conducted to assess the potential of phosphoric acid, dodecyl ester, sodium salt to induce chromosomal aberrations. Numerical, but not structural, chromosome aberrations were detected under the present experimental conditions.
| Purity | : | −% (Not analyze) |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 423 |
| Route | : | Oral(gavage) |
| Doses | : | 2000 mg/kg |
| Number of animals/group | : | Females, 3 |
| Vehicle | : | Olive oil |
| GLP | : | Yes |
Test results:
| Purity | : | −% (Not analyzed) |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral(gavage) |
| Dosage(main study) | : | 0(vehicle), 250, 500, 1000 mg/kg/day |
| Number of animals/group | : | Males, 17; females, 17(0 and 1000 mg/kg/day) Males, 12; females, 12(250 and 500 mg/kg/day) |
| Vehicle | : | Olive oil |
| Administration period | : |
Males, 42 days |
| Terminal killing | : |
Males, day 43 |
| GLP | : | Yes |
[Additional test]
| Dosage | : | 0(vehicle), 62.5, 125 mg/kg/day |
| Number of animals/group | : | Males, 6; females, 6 |
| Vehicle | : | Olive oil |
| Administration period | : | Males and females, 45 days |
Test results:
<Repeated dose toxicity>
Gross pathological examination at the end of the administration period revealed recessed areas in the forestomachs at 250 mg/kg and above and rough mucosa or white foci at 500 mg/kg and above, and erosion/ulceration, mucosal thickening and submucosal edema at 250 mg/kg and above on histopathological examination.
<Reproductive/developmental toxicity>
No test article-related effects were observed on the estrus cycle, precoital period, copulation index, insemination index or fertility index. No test article related-effects were observed in dams regarding the length of the gestation period, gestation index, number of corpora lutea, number of implantation sites, the implantation index, lactation behavior, stillbirth index, number of offspring born alive, live birth index, sex ratio, observation of live offspring at birth, necropsy findings at necropsy on day 4 after birth, body weights or viability of offspring.
Based on the results described above, the NOAEL of phosphoric acid, dodecyl ester, sodium salt with repeated administration was judged to be less than 250 mg/kg/day for both males and females, and the NOAEL for reproductive/developmental toxicity to be 1000 mg/kg/day for male and female parent animals and offspring.
An additional test was conducted in order to determine the NOAEL by repeated administration, where the test article was administered to male and female rats of the same strain at 62.5 or 125 mg/kg for 45 days. In the additional test, submucosal edema in the forestomach was observed in females at 125 mg/kg.
Based on the results of the main study and the additional test, the NOAEL of phosphoric acid, dodecyl ester, sodium salt by repeated administration was judged to be 125 mg/kg/day for males and 62.5 mg/kg/day for females.
3-1. Bacterial test 1)
| Purity | : | ― % (Not analyze) |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose solution |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98 and WP2 uvrA), sodium azide (TA1535) and 2-methoxy-6-chloro-9-[3-(2-chloroethyl)-aminopropylamino] acridine・2HCl (TA1537) +S9 mix; Benzo [a] pyrene (TA100, TA98, TA1537) and 2-aminoanthracene (TA1535 and WP2 uvrA) |
| Dosage | : | [Dose-range finding Test] -S9 mix; 0, 0.305, 1.22, 4.88, 19.5, 78.1, 313, 1250 and 5000 μg/plate (all strains) +S9 mix; 0, 0.305, 1.22, 4.88, 19.5, 78.1, 313, 1250 and 5000 μg/plate (all strains) [Main test] -S9 mix; 0, 156, 313, 625, 1250, 2500 and 5000 μg/plate (all strains) +S9 mix; 0, 156, 313, 625, 1250, 2500 and 5000 μg/plate (all strains) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| GLP | : | Yes |
No mutagenic activity was found in the S. typhimurium and E. coli strains. Toxicity was not observed in any strains, with or without metabolic activation.
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | −% (Not analyze) |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose solution |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 23, 45, 90, 180 and 360 μg/mL +S9 mix (short-term treatment); 0, 23, 45, 90, 180 and 360 μg/mL -S9 mix (continuous treatment 24 hr); 0, 11, 23, 45, 90 and 180 μg/mL -S9 mix (continuous treatment 48 hr); 0, 11, 23, 45, 90 and 180 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [*] | [ ] | [ ] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
Lowest concentration producing cytogenetic effects in vitro:ff
Without metabolic activation (continuous treatment 48 hr): 45 μg/mL (polyploidy)
| 1) | The tests were performed by Bozo Research Center Inc, 1284, Kamado, Gotemba-shi, Shizuoka, 412-0039, Japan. Tel +81-550-82-2000, Fax +81-550-82-2379 |