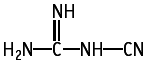

Cyanoguanidine was studied for oral toxicity in rats in an OECD preliminary reproduction toxicity screening test at doses of 0, 40, 200 and 1000 mg/kg/day.
With regard to repeat dose toxicity, the compound had no general toxicological effects in either sex. Thus the NOEL for repeat dose toxicity is considered to be 1000 mg/kg/day for both sexes. In terms of reproductive/developmental toxicity, the compound had no effects on any relevant parameters. The NOEL for reproductive/developmental toxicity is considered to be 1000 mg/kg/day for parental animals and offspring.
Cyanoguanidine was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
Cyanoguanidine did not induce structural chromosomal aberrations or polyploidy in CHL cells, with or without an exogenous metabolic activation system.
| Purity | : | 99.8 % |
| Test species/strain | : | Rats/Crj:CD(SD)IGS |
| Test method | : | OECD Preliminary Reproductive Toxicity Screening Test |
| Route | : | Oral(gavage) |
| Dosage | : | 0(Vehicle), 40, 200, 1000 mg/kg/day |
| Number of animals/group | : | Males, 12; females, 12 |
| Vehicle | : | 3 % gum arabic solution |
| Administration period | : | Males, 44 days Females, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Males, day 45 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
The compound had no effects on clinical signs, body weights, food consumption or necropsy findings. The organ weights of kidneys, testes and epididymides were similar among all four groups. No histopathological changes ascribable to the compound in these organs were found in either sex.
The NOEL for repeat dose toxicity is considered to be 1000 mg/kg/day for both sexes.
<Reproductive and developmental toxicity>
The compound had no effects on reproductive parameters such as the mating index, the fertility index, numbers of corpora lutea or implantations, the implantation index, the delivery index, the gestation index, gestation length, parturition or maternal behavior. On examination of neonates, there were no significant differences in numbers of offspring or live offspring, the sex ratio, the live birth index, the viability index or body weights. No abnormal findings ascribable to the compound were found for external features or clinical signs or on necropsy of the offspring.
The NOEL for reproductive and developmental toxicity is considered to be 1000 mg/kg/day for parental animals and offspring.
| Purity | : | 99.8 % |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537, E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan)and OECD Guidelines No. 471 and 472 |
| Procedures | : | Pre-incubation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98 and WP2 uvrA), Sodium azide(TA1535), 9-Aminoacridine hydrochloride(TA1537) +S9 mix; 2-Aminoanthracene(all strains) |
| Doses | : | -S9 mix; 156, 313, 625, 1250, 2500 and 5000 μg/plate +S9 mix; 156, 313, 625, 1250, 2500 and 5000 μg/plate |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
S. typhimurium TA100, TA1535, TA98 and TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.8 % |
| Type of cell used | : | Chinese hamster lung(CHL)cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan)and OECD Guideline No. 473 |
| Solvent | : | Saline |
| Positive controls | : | -S9 mix, Mitomycin C +S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix(continuous exposure): 0, 210, 420, 840 μg/mL -S9 mix(short-term exposure): 0, 210, 420, 840 μg/mL +S9 mix(short-term exposure): 0, 210, 420, 840 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki, 314-0255, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), Japan, 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |