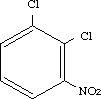

1,2-Dichloro-3-nitrobenzene was studied for oral toxicity in rats in a single dose toxicity test at doses of 190, 256, 435, 466, 630 and 850 mg/kg and in an OECD combined repeat dose and reproductive/developmental toxicity screening test at doses of 0, 1, 5, 25 and 100 mg/kg/day. 1,2-Dichloro-3-nitrobenzene was also tested for mutagenicity with assays for chromosomal aberrations in cultured Chinese hamster (CHL) cells.
The single dose oral toxicity test revealed LD50s of 381 mg/kg for male and 512 mg/kg for female rats.
For repeat dose toxicity, 1,2-dichloro-3-nitrobenzene showed toxicological effects on the liver and kidneys, and induced hemolytic anemia. Liver and kidney weights were increased and splenic hemosiderosis occurred. Hepatocellular swelling and hyaline droplets in the renal convoluted tubular epithelium were observed in males given 25mg/kg or more. Elevations in urine protein, serum Na, total protein and total cholesterol, and decreases in blood urea nitrogen and body weight gain were noted in males given 100 mg/kg or more. The females given 25 mg/kg or more exhibited similar pathological changes in the liver, kidneys and spleen. The change in the kidney was characterized by vacuolated tubular epithelium. NOELs for repeat dose toxicity were 5 mg/kg/day for both sexes. For reproductive/developmental toxicity, 1,2-dichloro-3-nitrobenzene did not show any significant effects on dams or pups. NOELs for reproductive performance and offspring development were 100 mg/kg/day in both sexes.
1,2-Dichloro-3-nitrobenzene induced chromosomal aberrations weakly without exogenous metabolic activation at concentrations of 0.12 mg/ml or more. Polyploidy was also induced after 48 h continuous treatment with this chemical.
| Purity | : | 99.15% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Doses | : | 190, 256,345, 466, 630, 850 mg/kg |
| Number of animals | : | Male, 5; female, 5/group |
| GLP | : | Yes |
| Test results | : | LD50: Male, 381 mg/kg; female, 512 mg/kg |
| Purity | : | 99.15 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 1, 5, 25, 100mg/kg/day |
| Number of animals | : | Male,10; Female,13/group |
| Vehicle | : | Olive oil |
| Administration period | : | Male, 44 days |
| Administration period | : | Male, 44 days Female, from 14 day before mating to day 3 of lactation |
| Terminal kill | : | Male, day 45 Female, day 4 of lactation |
| GLP | : | Yes |
The change in female kidneys was characterized by vacuolated tubular epithelium. Incidence of atrophic thymus was increased in the 100 mg/kg dose group when compared with the control group. There were no notable alterations in general conditions, and no fatalities among any of the dosage groups. Food consumption of the 100 mg/kg males and females was decreased on the first day of the treatment but showed no significant difference from control values thereafter.
<Reproductive and developmental toxicity>
The test substance was found not to show toxicity effects in the reproductive and developmental toxicity test.
| Purity | : | 99.15 % |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guideline for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | DMSO |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9: 0, 0.03, 0.06, 0.12 mg/ml +S9: 0, 0.024, 0.049, 0.097 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Lowest concentration producing cytogenetic effects in vitro:
with metabolic activation: > 0.097 mg/ml
without metabolic activation: 0.12 mg/ml
Genotoxic effects:
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [*] | [ ] | [ ] |
| 1) | The tests were performed by the Research Institute for Animal Science in Biochemistry and Toxicology, 3-7-11 Hashimotodai, Sagamihara-shi, Kanagawa, 229, Japan. Tel 81-427-62-2775 Fax 81-427-62-7979 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |