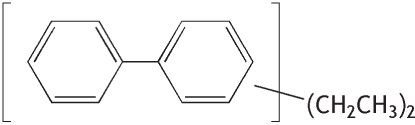
Molecular formula: C16H18 Molecular weight: 210.31

A repeated dose oral toxicity study of diethylbiphenyl was conducted in rats according to OECD Test Guideline 407, at doses of 0, 15, 60 and 240 mg/kg. On hematological and biochemical examinations at the end of the dosing period, prolonged partial prothrombin and activated partial thromboplastin times were observed in males and the total cholesterol level was increased in females of the 240 mg/kg group. Liver weights in females and relative liver weights in both sexes were significantly increased in the 240 mg/kg group. Histological examination revealed centrilobular hypertrophy of hepatocytes in males of the 240 mg/kg group.
From these results, the NOAEL is considered to be 60 mg/kg/day for both sexes.
Genotoxicity of diethylbiphenyl was studied by a reverse mutation test in bacteria. This substance was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
Genotoxicity of diethylbiphenyl was studied by a chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells with Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD Test Guideline 473. Diethylbiphenyl did not induce structural chromosomal aberrations or polyploidy under the present test conditions, with or without metabolic activation.
| Purity | : | 97.74 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 407 |
| Route | : | Oral(gavage) |
| Dosage | : | 0(vehicle), 15, 60, 240 mg/kg/day |
| Number of animals/group | : | Males, 10; females, 10(0, 240 mg/kg) Males, 5; females, 5(15, 60 mg/kg) |
| Vehicle | : | Corn oil |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Males and females, days 29 or 43 |
| GLP | : | Yes |
In the 240 mg/kg group, transient salivation immediately after administration was observed frequently in both sexes after the 9th day of observation. On hematological and biochemical examinations at the end of the dosing period, prolonged partial prothrombin and activated partial thromboplastin times in males and increased total cholesterol levels in females were observed in the 240 mg/kg group. Liver weights in females and relative liver weights in both sexes were increased at 240 mg/kg, and histological examination revealed centrilobular hypertrophy of hepatocytes in the males.
At the end of the recovery period, the above changes observed at the end of the dosing period had disappeared.
From these results, the NOAEL is considered to be 60 mg/kg/day for both sexes.
2. Genetic Toxicity
2-1. Bacterial test 1)
| Purity | : | 97.74 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA),
sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9 mix; 2-Aminoanthracene (five strains) |
| Dosage | : | -S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (five strains) +S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (TA100, TA1537, WP2 uvrA), 0, 78.1, 156, 313, 625, 1250, 2500, 5000 μg/plate (TA1535), 0, 39.1, 78.1, 156, 313, 625, 1250, 2500, 5000 μg/plate (TA98) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3(1 for cytotoxicity test) |
| Number of replicates | : | 2(plus 1 cytotoxicity test) |
| GLP | : | Yes |
Test results:
This chemical did not induce gene mutations in S. typhimurium TA100, TA98, TA1535, TA1537 or E. coli WP2 uvrA strains with or without S9 mix. Growth inhibition was not observed up to 5000 μg/plate in any strain, with or without S9 mix.
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 97.74 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9; Mitomycin C +S9; Cyclophosphamide |
| Dosages | : | -S9 (short-term treatment); 0, 0.013, 0.020, 0.030 mg/mL +S9 (short-term treatment); 0, 0.056, 0.11, 0.23 mg/mL -S9 (continuous treatment); 0, 0.013, 0.020, 0.030 mg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
The incidence of cells with structural chromosomal aberrations and polyploidy was not statistically or biologically significant at any dose in either test system.
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751, Fax +81-463-82-9627 |