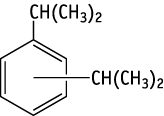

Diisopropylbenzene was studied for oral toxicity in rats in a reproduction/developmental toxicity screening test at doses of 0, 6, 30, 150 and 750 mg/kg/day (12 animals of each sex per group).
In the males, exophtalmos was noted in 2 animals at 750 mg/kg. Transiently lowered food consumption was also noted in the 750 mg/kg group. No changes caused by the substance were noted in terms of body weight, necropsy findings, organ weights, or sperm examination. On histopathological examination, vacuolization of lens fibers and hyperplasia of epithelium lentis were noted in 2 and 1 animal, respectively, of the 750 mg/kg group.
In the females, mydriasis was noted in the 750 mg/kg group. Transiently lowered body weight were evident in the 750 mg/kg group during the pregnancy period and transiently reduced food consumption was noted before mating. No changes caused by the substance were noted with regard to necropsy, organ weights, or histopathological examination.
The NOELs for repeat dose toxicity are considered to be 150 mg/kg/day for both sexes.
Regarding reproductive/developmental toxicity, no changes caused by the substance were noted in terms of the copulation index, gestation length, delivery conditions, nursing conditions, fertility index, number of corpora lutea, implantation rate, or gestation index.
The NOELs for reproductive performance are considered to be 750 mg/kg/day for both sexes.
Regarding the pups, no changes caused by the substance were noted in the number of pups, number of stillbirths, number of live pups born, sex ratio, delivery index, birth index, live birth index, viability index, or body weight.
The NOEL for pup development is considered to be 750 mg/kg/day.
| Purity | : | 98.3 % |
| Test species/strains | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral (gavage) |
| Doses | : | 0(vehicle), 6, 30, 150, 750 mg/kg/day |
| Number of animals/group | : | Males, 12; females,12 |
| Vehicle | : | Corn oil |
| Administration period | : | Males, 50-52 days Females, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Males, days 51-53 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
<Repeat dose toxicity>
<Reproductive and developmental toxicity>
| 1) | The test was performed by Nihon Bioresearch Inc., 6-104 Majima, Fukuju-cho, Hashima, Gifu, 501-6251, Japan. Tel +81-58-392-6222 Fax +81-58-392-1284 |