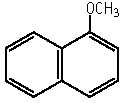
1-Methoxynaphthalene
1-メトキシナフタレン
[CAS No. 2216-69-5]
Molecular formula: C11H10O Molecular weight: 158.20
ABSTRACT
1-Methoxynaphthalene was studied for its oral toxicity in rats in a single dose toxicity test at doses of 0, 500, 1000 and 2000 mg/kg. The single dose oral toxicity test revealed LD50 values of 2000 mg/kg or more for males and about 2000 mg/kg for females.
1-Methoxynaphthalene was studied for its oral toxicity in rats in a 28-day repeat dose toxicity test at doses of 0, 30, 100, 300 and 1000 mg/kg/day. In the males, salivation and hyaline droplet degeneration of renal tubules were observed at doses of 100 mg/kg or more. Soiling of hair, suppression of body weight gain, decreased food consumption and, RBC, hemoglobin, hematocrit, MCHC and total protein levels, increases in reticulocytes, total bilirubin, potassium, the absolute and relative spleen weights and relative kidney weight, darkening of the spleen with hemosiderin deposition and congestion, as well as hemosiderin deposition in the liver were seen in animals given 300 mg/kg or more. In addition ptosis and increased water consumption, urine volume, methemoglobin, GPT and the relative liver weights were seen at 1000 mg/kg. For the females, salivation and hemosiderin deposition in the spleen and liver were seen with doses of 100 mg/kg or more, along with soiling of hair discoloration, ptosis, lacrimation, suppression of body weight gain, decreases in food consumption, RBC, hemoglobin, hematocrit and MCHC, increases in water consumption, reticulocytes, total bilirubin, darkening of the spleen and relative weights of the liver and kidneys at 300 mg/kg or more. Increased urine volume, methemoglobin, GPT, potassium, absolute liver and kidney weights, absolute and relative weights of the spleen and congestion in the spleen were seen in the 1000 mg/kg group. The NOEL for repeat dose toxicity is considered to be 30 mg/kg/day for both sexes.
1-Methoxynaphthalene was not mutagenic to Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA, with or without an exogeneous metabolic activation system.
1-Methoxynaphthalene induced structural chromosomal aberrations in CHL/IU cells in the presence of an exogenous metabolic activation system. Polyploid cells were not induced under the present experimental conditions.
SUMMARIZED DATA FROM THE STUDIES
1. Single Dose Oral Toxicity 1)
| Purity | : | 99.1% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 500, 1000, 2000 mg/kg/day |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Corn oil |
| GLP | : | Yes |
Test results:
- Two females of the 2000 mg/kg group died. Both sexes of the 500,1000 mg/kg and 2000 mg/kg groups showed decrease in locomotor activity and ptosis on the day of administration. Lacrimation was additionally observed in males and females receiving the highest dose on the day of administration. Body weight gains of both sexes of the 1000 and 2000 mg/kg groups tended to be suppressed. Based on the above results, the LD50 value of 1-methoxynaphthalene is considered to be 2000 mg/kg or more for males and about 2000 mg/kg for females.
2. Repeat Dose Toxicity 1)
| Purity | : | 99.1% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for the 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 30, 100, 300, 1000 mg/kg/day |
| Number of animals/group | : | Males, 10 and 15 (0 and 1000 mg/kg/day)
Females, 10 and 15 (0 and 1000 mg/kg/day) |
| Vehicle | : | Corn oil |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Males and females, days 29 or 43 (0, 1000 mg/kg) |
| GLP | : | Yes |
Test results:
- No deaths occurred in any group during the treatment period. Salivation was seen in both sexes of the groups treated with 100 mg/kg or more, and soiling of hair was seen in both sexes of the groups treated with 300 mg/kg or more. Ptosis was seen in females of the groups treated with 300 mg/kg or more and in the males of the 1000 mg/kg group. Lacrimation was seen in the females of the groups treated with 300 mg/kg or more.
- Suppression in body weight gain was seen in both sexes of the groups treated 300 mg/kg or more.
- Decreased food consumption was noted for both sexes of the groups treated with 300 mg/kg or more. These changes disappeared during the recovery period.
- Increased water consumption was noted for females of the 300 mg/kg group and in both sexes of the 1000 mg/kg group.
- On urinalysis, both sexes of the 1000 mg/kg group showed increases in urine volume and numbers of animals showing acidity. Slight or moderate increases in bilirubin were noted in the 1000 mg/kg males. All these disappeared before termination of the recovery period.
- On hematological examination, both sexes of the groups treated with 300 mg/kg or more showed decreases in RBC, hemoglobin, hematocrit and MCHC and increased methemoglobin. All these changes has disappeared by the termination of the recovery period.
- On blood chemical examination, both sexes of the groups treated with 300 mg/kg or more showed increases in total bilirubin, the males of the groups treated with 300 mg/kg or more showed decreases in total protein and increases in potassium, both sexes of the 1000 mg/kg group showed increases in GPT, and the females of the 1000 mg/kg group showed increase in potassium. All these changes had disappeared by the termination of the recovery period.
- At necropsy, darkening of spleens were found in both sexes of the groups treated with 300 mg/kg or more. This change had disappeared by the termination of the recovery period.
- In the males, increases in the absolute and relative spleen weights and the relative kidney weight were observed in the groups treated with 300 mg/kg or more, along with increased relative liver weights in the 1000 mg/kg group. In the females, increases in the relative liver and kidney weights were observed in the groups treated with 300 mg/kg or more, and increases in the absolute liver and kidney weights and the absolute and relative spleen weights were noted for the 1000 mg/kg group. These changes had disappeared by the termination of the recovery period.
- Histopathological examination of the males revealed hyaline droplet degeneration of renal tubules in the groups treated with 100 mg/kg or more, and congestion in the spleen and hemosiderin deposition in the liver were found in the groups treated with 300 mg/kg or more. In the females, hemosiderin deposition in the spleen and liver were found in the groups treated with 100 mg/kg or more, and congestion in the spleen was found in the 1000 mg/kg group. At the termination of the recovery period, hemosiderin deposition in the spleen and liver was still found in both sexes of the 1000 mg/kg group, and in the liver was found in the females of the 1000 mg/kg group. The above results suggested that 1-methoxynaphthalene exerts effects on general condition, body weight, food consumption, water consumption, blood, liver, spleen and kidneys. The NOEL for 1-methoxynaphthalene administered orally for 28-days is considered to be 30 mg/kg/day for both sexes.
3. Genetic Toxicity
3-1. Bacterial test 2)
| Purity | : | 98 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD (471 and 472) |
| Procedures | : | Plate incorporation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix, 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, WP2, TA98), Sodium azide (TA1535) and 9-Aminoacridine (TA1537),
+S9 mix, 2-Aminoanthracene (five strains) |
| Dosage | : | -S9 mix, 0, 7.81, 15.6, 31.3, 62.5, 125 and 250 μg/plate (TA100, TA1535 and TA1537), 0, 15.6 - 500 μg/plate (TA98) and 0, 156 - 5000 μg/plate (WP2)
+S9 mix, 0, 7.81 - 250 (TA1537), 0, 15.6 - 500 μg/plate (TA100 and TA1535) , 0, 31.3 - 1000 μg/plate (TA98) and 0, 78.1 - 2500 μg/plate (WP2) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
- Test results:
- This chemical did not induce gene mutations in the S. typhimurium and E. coli strains. Toxicity was observed at 125 μg/plate (TA100 and TA1537), 150 μg/plate (TA1535), 250 μg/plate (TA98) and 5000 μg/plate (WP2) without S9 mix, and 150 μg/plate (TA1537), 250 μg/plate (TA1535), 500 μg/plate (TA100 and TA98) and 1500 μg/plate (WP2) with S9 mix.
- Genetic effects:
- Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
- Escherichia coli WP2 uvrA
| + | ? | - |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
3-2. Non-bacterial in vitro test (chromosomal aberration test) 2)
| Purity | : | 98% |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Dimethylsulfoxide |
| Positive controls | : | -S9 mix, Mitomycin C
+S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix (continuous treatment): 0, 0.020, 0.040, 0.080 mg/ml
-S9 mix (short-term treatment): 0, 0.010, 0.020, 0.040 mg/ml
+S9 mix (short-term treatment): 0, 0.005, 0.010, 0.020 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
- Test results:
- Were induced in the presence of an exogenous metabolic activation system. Structural chromosomal aberrations.
- Lowest concentration producing cytogenetic effects in vitro:
- with metabolic activation (short-term treatment): 0.020 mg/ml (clastogenicity)
- Genotoxic effects:
| clastogenicity | polyploidy |
| + | ? | - | + | ? | - |
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| with metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The test was performed by Nihon Bioresearch Inc. Hashima Laboratory, 6-104 Majima, Fukuju-cho, Hashima, Gifu, 501-62 Japan Tel +81-58-392-6222 Fax +81-58-391-3171 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |

