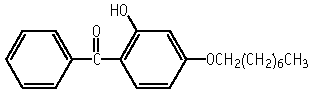

2-Hydroxy-4-(octyloxy)benzophenone was not mutagenic to Salmonella typhimurium, TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA. Neither structural nor numerical chromosomal aberrations were induced in CHL/IU cells up to the limit concentration of 5 mg/ml, in the absence or presence of an exogeneous metabolic activation system.
| Purity | : | > 99% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Testing for Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 20, 140, 1000 mg/kg/day |
| Number of animals/group | : | Males, 6 ; females, 6 |
| Vehicle | : | 0.5% sodium carboxymethyl cellulose solution containing 0.1% Tween 80 |
| Administration period | : | Males and females, 28 days |
| Terminal kill | : | Days 29 or 43 |
| GLP | : | Yes |
Test results:
| Purity | : | ≧ 99% |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test methods | : | OECD guideline (No. 471, 472) and Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Pre-incubation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix, AF-2 (TA100, TA98), sodium azide (TA1535), ENNG (WP2 uvrA) and 9-aminoacridine (TA1537) +S9 mix, 2-aminoanthracene (all strains) |
| Doses | : | 313, 625, 1250, 2500, 5000μg/plate |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
| + | ? | - | |
| without metabolic activation | [ ] | [ ] | [*] |
| with metabolic activation | [ ] | [ ] | [*] |
| + | ? | - | |
| without metabolic activation | [ ] | [ ] | [*] |
| with metabolic activation | [ ] | [ ] | [*] |
| Purity | : | ≧99% |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | OECD guideline (No. 473) and Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9 mix, Mitomycin C +S9 mix, Benzo[a]pyrene |
| Doses | : | -S9 mix (24 h treatment): 0, 1250, 2500, 5000 μg/ml -S9 mix (48 h treatment): 0, 1250, 2500, 5000 μg/ml -S9 mix (6 h pulse treatment): 0, 1250, 2500, 5000 μg/ml +S9 mix (6 h pulse treatment): 0, 1250, 2500, 5000 μg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki, 314-02, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |