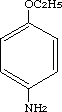

4-Ethoxybenzenamine was studied for oral toxicity in rats in a 28-day repeat dose test at doses of 0, 10, 40 and 160 mg/kg/day and in an OECD preliminary reproduction toxicity screening test at doses of 0, 3, 12, 50, and 200 mg/kg/day. 4-Ethoxybenzenamine was also tested for mutagenicity with assays for reverse mutation in bacteria, chromosomal aberrations in cultured Chinese hamster (CHL) cells and micronuclei induction in mice.
In the repeat dose toxicity test, hematological and urinary examinations revealed a decrease in erythrocyte counts, increases in reticulocytes and urinary urobilinogen in the 40 and160 mg/kg groups, and methemoglobinemia in the 160 mg/kg group of both sexes. An increase in spleen weight was noted in both sexes treated with 40 mg/kg or more. Histopathological examinations in these groups showed hemosiderosis, increased extramedullary hematopoiesis, congestion of the spleen and myeloid hyperplasia of the bone marrow. The NOEL for repeat dose toxicity was 10 mg/kg/day.
In the preliminary reproduction toxicity screening test, 4-Ethoxybenzenamine caused salivation, a decrease in food consumption in males and spleen hypertrophy in both sexes at doses of 50 mg/kg or more. The 200 mg/kg females showed cyanosis and delay of delivery. Nine out of 12 dams in the 200 mg/kg dose died on days 23 to 25 of pregnancy. This chemical is considered to affect dams in the perinatal period and neonatal development in spite of no changes in copulation and fertility indexes. NOELs for reproductive performance were 200 mg/kg/day in males, and 50 mg/kg/day in females. The NOEL for offspring development was 50 mg/kg/day.
4-Ethoxybenzenamine was not mutagenic to bacteria with and without exogenous metabolic activation up to 5000 μg/plate. However, the chemical induced chromosomal aberrations in CHL cells with and without exogenous metabolic activation but not polyploidy under the test condition. It also induced micronucleated polychromatic erythrocytes at the dose of 1000 mg/kg in female BDF1 mice.
| Purity | : | 99.36% |
| Test species/strain | : | Rat/Crj:F344 |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage ) |
| Doses | : | 0 (vehicle), 10, 40, 160 mg/kg/day |
| Number of animals | : | Male, 5; Female, 5/group |
| Vehicle | : | Olive oil |
| Administration period | : | Male and Female, 28days |
| Terminal kill | : | Days 29 or 43 |
Test results:
| Purity | : | > 99 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Preliminary Reproduction Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 3, 12, 50, 200 mg/kg/day |
| Number of animals | : | Male, 12; Female, 12/group |
| Vehicle | : | Corn oil |
| Administration period | : | Male, 49 days Female, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Male, day 50 Female, day 4 of lactation |
| GLP | : | Yes |
Test results:
| Purity | : | 99.36 % |
| Test species/strains | : | S. typhimurium TA100, TA98, TA102, TA97 |
| Test method | : | Maron & Ames (1983) |
| Procedure | : | Preincubation assay |
| Solvent | : | DMSO |
| Positive controls | : | -S9, AF-2 (TA100, TA98) ICR-191 (TA97) Mitomycin C (TA102) +S9, 2-Aminoanthracene (all strains) |
| Dose | : | 0, 25, 50, 100, 250, 500, 1,000, 2,500, 5,000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| No. of replicate | : | 1 |
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.36 % |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guideline for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | DMSO |
| Dose | : | -S9mix: 0, 0.01, 0.02, 0.05 mg/ml +S9mix: 0, 0.05, 0.1, 0.2 mg/ml |
| S-9 | : | Rat liver, induced by Phenobarbital and 5.6-Benzoflavone |
| Plates/test | : | 1 |
| + | ? | - | |
| with metabolic activation: | [*] | [ ] | [ ] |
| without metabolic activation: | [*] | [ ] | [ ] |
| Purity | : | > 98 % |
| Test species/strain | : | Mouse/Crj:BDF1 |
| Test method | : | OECD Test Guideline 474 |
| Doses | : | Male: 0, 150, 300, 600 mg/kg Female: 0, 250, 500, 1000 mg/kg |
| Solvent | : | Olive oil |
| GLP | : | Yes |
| + | ? | - | |
| Micronucleus test: | [*] | [ ] | [ ] |
| 1) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel. 81-463-82-4751 Fax 81-463-82-9627 |
| 2) | The test was performed by Nihon Bioresearch Inc., Hashima Laboratory, 6-104 Majima, Fukuju-cho, Hashima, Gifu, 501-62, Japan. Tel 81-583-92-6222 Fax 81-583-92-1284 |
| 3) | The test was performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |