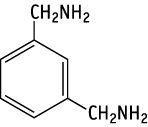

1,3-Bis(aminomethyl)benzene was studied for oral toxicity in rats in a reproduction/
developmental toxicity screening test at doses of 0, 50, 150 and 450 mg/kg/day.
In the repeat dose study, one male given 150 mg/kg, and three males and one female given 450 mg/kg died during the administration period. Salivation, noisy, breathing irregular respiration, abdominal distension and nasal discharge were observed in males given 150 mg/kg and in both sexes given 450 mg/kg. Suppression of body weight gain and decrease of food consumption were observed in both sexes given 450 mg/kg. Histpathologically, ulceration and squamous hyperplasia of the fore-stomach were observed in both sexes given 450 mg/kg. There were no adverse effects of the test substance on reproductive organs in either sex.
NOELs for repeat dose toxicity are considered to be 50 mg/kg/day for males, and 150 mg/kg/day for females, respectively.
In terms of reproductive/developmental toxicity, the test substance showed no effects on any relevant parameters.
NOELs for reproductive and developmental toxicity are considered to be 450 mg/kg/day for both parental animals and offspring.
| Purity | : | 99.80 wt% |
| Test species/strains | : | Rat/Crj:CD(SD) |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral(gavage) |
| Doses | : | 0(vehicle), 50, 150, 450 mg/kg/day |
| Number of animals/group | : | Males, 12; females, 12 |
| Vehicle | : | Purified Water |
| Administration period | : | Males, 48 days Females, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Males, day 48 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
Deaths occurred of one male receiving 150 mg/kg, and three males and one female receiving 450 mg/kg. Salivation was observed in males in groups receiving 150mg/kg or more and in females in groups receiving 450mg/kg. Furthermore, treatment-related clinical signs, like nasal, rattle, irregular respiration, abdominal distension and nasal discharge were noted in the same groups. Body weight gain and food consumption were decreased in animals receiving 450 mg/kg.
No test-compound related differences were observed in organ weights. As a gross necropsy finding, ulceration of the stomach was observed and histpathologically ulceration and squamous hyperplasia of the forestomach were observed in both sexes given 450 mg/kg. There were no adverse effects of the test substance on the reproductive organs in either sex.
NOELs for repeat dose toxicity are considered to be 50 mg/kg/day for males, and 150 mg/kg/day for females, respectively.
<Reproductive and developmental toxicity>
Regarding the reproductive ability of parent animals, no effects were detected in terms of the copulation index, fertility index, gestation length, number of corpora lutea or implantations, implantation index, gestation index, delivery index and, parturition or maternal behavior. There were no significant differences in numbers of offspring or live offspring, the sex ratio, the live birth index, the viability index or body weights. No abnormal findings related to the test substance were noted on external examination, with respect to clinical signs, or on necropsy of the offspring.
NOELs for reproductive and developmental toxicity are considered to be 450 mg/kg/day for both parental animals and offspring.
| 1) | The test was performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |