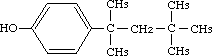

p-tert-Octylphenol was studied for oral toxicity in rats in a 28-day repeat dose toxicity test at doses of 0, 15, 70 and 300 mg/kg/day and for mutagenicity with assays for reverse mutation in bacteria and chromosomal aberrations in cultured Chinese hamster (CHL) cells.
In the repeat dose toxicity study, p-tert-octylphenol caused salivation in both sexes, a decrease in the body weight gain in males and an increase in water intake in both sexes of the high dose group. Blood chemical analyses revealed an increase in Na in both sexes, and decreases in cholesterol and the A/G ratio and increases in BUN and triglyceride, in females of the medium- or high-dose group. The high dose group demonstrated increased urine volumes and urinalysis showed decreases in specific gravity, Na, Cl and K. Increases in organ weights of the kidney and liver were found in the high dose group. Pathological examination revealed grayish kidney patches and regeneration of renal tubules. However, changes attributable to p-tert-octylphenol disappeared or tended to recover after discontinuation of administration. The NOELs for repeat dose toxicity test was 15 mg/kg/day.
p-tert-Octylphenol was not mutagenic for S. typhimurium TA100, TA1535, TA98, TA1537, and E. coli WP2 with or without exogenous metabolic activation up to 5000 μg/plate. This chemical induced neither chromosomal aberrations nor polyploidy with or without exogenous metabolic activation up to the cytotoxic concentration.
| Purity | : | 98.24 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 15, 70, 300 mg/kg/day |
| Number of animals | : | Male, 6; Female, 6/group |
| Vehicle | : | Olive oil |
| Administration period | : | Male and Female, 28 days |
| Terminal kill | : | Male and Female, days 29 or 43 |
| GLP | : | Yes |
The test substance did not cause any consistent changes in food consumption and hematological parameters. For the high dose group, blood chemical analyses showed an increase in Na for females and males, a decrease in cholesterol and an increase in BUN and triglyceride for females, and a decrease in albumin in females. A/G ratios were lowered in the medium- and high- dose. Increased urine volume was evident in females and males at the high dose. Urinalysis showed decreases in specific gravity and in Na, Cl and K. Slight but statistically significant increases in kidney weights were found in the high-dose males and females and in liver weights in the high-dose females.
The high-dose males and females showed grayish kidney patch as gross findings, and regeneration of renal tubules as microscopic findings. All changes attributable to the test substance disappeared or tended to recover after discontinuing the administration.
| Purity | : | > 97% |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedure | : | Plate method |
| Solvent | : | DMSO |
| Positive controls | : | -S9, AF-2 (TA100,WP2uvrA,TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-Aminoanthracene (all strains) |
| Doses | : | S. typhimurium: 0,1.56, 3.13, 6.25, 12.5, 25, 50, 100, 200 μg/plate E. coli: 0, 125, 250, 500, 1000, 2000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Genotoxic effects:
S. typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Puriy | : | > 97 % |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guideline for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | DMSO |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9: 0, 0.004, 0.008, 0.016 mg/ml +S9: 0, 0.010, 0.020, 0.040 mg/ml |
| S-9 | : | Rat liver, induced by phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Lowest concentration producing cytogenetic effects in vitro:
with metabolic activation: > 0.04 mg/ml
without metabolic activation: > 0.16 mg/ml
Genotoxic effects:
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| 1) | The test was performed by the Mitsubishi-Kasei Institute of Toxicological and Environmental Sciences, 14 Sunayama, Hazaki-machi, Kashima-gun, Ibaraki, 314-02, Japan. Tel 81-479-46-2871 Fax 81-479-46-2874 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |