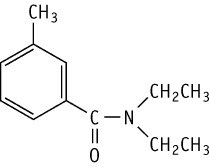

Oral toxicity was studied in rats according to the OECD Test Guideline 422 at doses of 0, 60, 200, and 600 mg/kg.
In the repeated dose oral toxicity test, salivation was observed in both sexes receiving 600 mg/kg. Decreased spontaneous motor activity, muscle weakness, ptosis of eyelids, and suppression of body weight gain were observed in females receiving 600 mg/kg. Blood chemistry determination revealed elevated total cholesterol in both sexes receiving 200 and 600 mg/kg and blood glucose in females receiving 600 mg/kg as compared to the control values. Urinalysis revealed a higher urine volume in all treated males and the pH of the urine in the 200 and 600 mg/kg groups was weakly acidic. Macroscopic examination revealed enlarged livers in males in all treatment groups, and in females receiving 200 and 600 mg/kg. Enlarged kidneys were also observed in males in all treatment groups. Histological evaluation revealed hypertrophy of hepatocytes and follicular cell hyperplasia of the thyroid gland in both sexes. The goblet cells of the large intestine were decreased in number in males receiving 600 mg/kg.
The NOEL for repeated dose oral toxicity test is considered to be less than 60 mg/kg/day for males, and 60 mg/kg/day for females.
In terms of reproductive/developmental toxicity, the test substance showed no adverse effects on any relevant parameters.
The NOEL for the reproductive and developmental toxicity test is considered to be 600 mg/kg/day for both parental animals and offspring.
Genotoxicity of N,N-diethyl-m-toluamide was studied by a reverse mutation test in bacteria and by a chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells.
N,N-Diethyl-m-toluamide was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 or Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
N,N-Diethyl-m-toluamide did not induce chromosomal aberrations in CHL/IU cells, with or without an exogenous metabolic activation system.
| Purity | : | 99.4 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 1000, 1300, 1700, 2000 mg/kg/day |
| Number of animals/group | : | Males and females; 5 |
| Vehicle | : | Corn oil |
| GLP | : | Yes |
Test results:
| Purity | : | 99.4 % |
| Test species/strains | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 60, 200, 600 mg/kg/day |
| Number of animals/group | : | Males, 12; females, 12 |
| Vehicle | : | Corn oil |
| Administration period | : | Males, 48 days Females, from 14 days before mating to day 4 of lactation |
| Terminal killing | : | Males, day 49 Females, day 5 of lactation |
| GLP | : | Yes |
Test results:
All animals survived the duration of the study. Salivation was temporarily observed in both sexes receiving 600 mg/kg. Decreased spontaneous motor activity, muscle weakness, and ptosis of eyelids were observed in females receiving 600 mg/kg during the lactation period, and suppression of central nervous system activity related to treatment with the test substance was suggested. Body weight gain was suppressed in females receiving 600 mg/kg from the late stage of the gestation period. No treatment-related effects were observed for food consumption or hematological parameters. Blood chemistry determination revealed higher total cholesterol in males and females receiving 200 and 600 mg/kg and blood glucose in females receiving 600 mg/kg than the control values. Urinalysis in males revealed a higher urine volume in all treatment groups, and the pH of urine in the 200 and 600 mg/kg groups was weakly acidic. Macroscopic examination revealed enlarged livers in males in all treatment groups, and in females receiving 200 and 600 mg/kg. Enlarged kidneys were observed in males in all treatment groups. Histological evaluation revealed hypertrophy of hepatocytes and follicular cell hyperplasia of the thyroid gland, and hypertrophy of zona glomerulosa in the adrenal glands in both sexes. The goblet cells of the large intestine were decreased in number in males receiving 600 mg/kg.
The NOEL for the repeated dose oral toxicity test is considered to be less than 60 mg/kg/day for males and females.
<Reproductive and developmental toxicity>
No adverse effects were observed on the estrus cycle, copulation, fertility results, delivery conditions, gestation length, number of corpora lutea or implantations, sex ratio, implantation index, gestation index, live birth index, or delivery index.
No treatment-related abnormal findings were noted on external observation, for clinical signs, viability, growth, or necropsy of the offspring.
The NOEL for the reproductive and developmental toxicity test is considered to be 600 mg/kg/day for both parental animals and offspring.
| Purity | : | 99.4 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2uvrA), Sodium azide (TA1535) and 9-Amino-acridine (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 78.1, 156, 313, 625, 1250, 2500 μg/plate (TA98, TA100, TA1535, TA1537) -S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (WP2 uvrA) +S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (all strains) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.4 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 1-Methyl-3-nitro-1-nitrosoguanidine +S9 mix; 3,4-Benzo[a]pyrene |
| Dosage | : | -S9 mix (6 hr short-term treatment); 0, 125, 250, 500, 750, 1000 μg/mL +S9 mix (6 hr short-term treatment); 0, 250, 500, 1000, 1500, 2000 μg/mL -S9 mix (24 hr continuous treatment); 0, 62.5, 125, 250, 500, 750, 1000 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Cytotoxicity was observed at 1000 μg/mL and at above 1500 μg/mL after 6 hr short-term treatment without and with S9 mix, and observed at above 750 μg/mL after 24 hr continuous treatment without S9 mix.
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were conducted at the Biosafety Research Center, Foods, Drugs and Pesticides (An-Pyo Center), 582-2, Arahama Shioshinden Fukude-Cho Iwata-Gun, Shizuoka 437-1213, Japan. Tel +81-538-58-1266, Fax +81-538-58-1393 |
| 2) | The tests were performed by the Research Institute for Animal Science in Biochemistry and Toxicology, 3-7-11 Hashimotodai, Sagamihara-shi, Kanagawa, 229-1132, Japan. Tel +81-42-762-2775Fax +81-42-762-7979 |