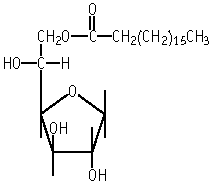

| Purity | : | unknown |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | 0.5% Carboxymethylcellulose sodium solution |
| Positive controls | : | -S9 mix, Mitomycin C +S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix (continuous treatment): 0, 0.020, 0.040, 0.080 mg/ml -S9 mix (short-term treatment): 0, 0.13, 0.25, 0.50 mg/ml +S9 mix (short-term treatment): 0,1.1, 2.2, 4.3 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| Without metabolic activation (short-term treatment): | 0.13 mg/ml (polyploidy) |
| With metabolic activation (short-term treatment): | 1.1 mg/ml (clastogenicity) |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] | [*] | [ ] | [ ] |
| with metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |