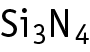
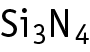
Silicon nitride was studied for oral toxicity in rats in a 28-day repeated dose toxicity test at doses of 0, 100, 300, and 1000 mg/kg/day.
Neither dead nor moribund animals were noted in either sex in any group. No changes attributable to the test substance were noted in general signs, body weights, food consumption, water intake, urinalysis, hematological examination, blood chemical analysis, necropsy findings, organ weights, or histopathological findings.
The NOEL for the 28-day repeated dose toxicity of silicone nitride is considered to be 1000 mg/kg/day for both sexes.
Silicone nitride was studied for oral toxicity in rats in an OECD preliminary reproduction toxicity screening test at doses of 0, 100, 300 and 1000 mg/kg/day.
With regard to repeated dose toxicity, no deaths were observed in any group. No effects related to administration of the test substance were observed on clinical signs, body weight, food consumption, necropsy, organ weight or histopathological examination in any treatment group. The NOEL is therefore considered to be 1000 mg/kg/day for repeated dose toxicity.
In terms of reproductive/developmental toxicity, there were no effects related to administration of the test substance on the reproductive performance of the parents or development of the next generation. The NOEL is therefore considered to be 1000 mg/kg/day for reproductive performance of the parents and for development of the offspring.
Silicone nitride was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA/pKM101, with or without an exogenous metabolic activation system.
Silicone nitride did not induce structural chromosomal aberrations nor polyploidy in CHL/IU cells with or without an exogenous metabolic activation system.
| Purity | : | ca. 97 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 250, 500, 1000, 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose |
| GLP | : | Yes |
Test results:
| Purity | : | ca. 97 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | Guideline for the 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 100, 300, 1000 mg/kg |
| Number of animals/group | Males, 12; females, 12 (0, 300, 1000 mg/kg) Males, 6; females, 6 (100 mg/kg) | |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Males and females, day 29 or 43 |
| GLP | : | Yes |
Test results:
The NOEL for the 28-day repeated dose toxicity of silicone nitride is considered to be 1000 mg/kg/day for both sexes.
| Purity | : | ca. 97.0 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 421 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 100, 300, 1000 mg/kg/day |
| Number of animals/group | Males, 12; females, 12 | |
| Vehicle | : | 0.5 % Sodium carboxymethylcellulose |
| Administration period | : | Males, 49 days Females, from 14 days before mating to day 3 of lactation |
| Terminal killing | : | Males, day 50 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
No deaths occurred in any group. There were no changes in clinical signs, body weight, food consumption, necropsy, organ weight or histopathological examination in the silicone nitride groups. The NOEL for repeated dose toxicity is considered to be 1000 mg/kg/day for males and females.
<Reproductive and developmental toxicity>
As concerns reproductive performance, no effects related to administration of the test substance were observed on the estrous cycle, numbers of corpora lutea or implantations, copulation index or fertility indices for the males or females. On examination at delivery and during the lactation period, no effects related to administration of the test substance were observed on the gestation days, number of litters or live newborn, gestation index, stillbirth index, birth index, sex ratio, body weights of offspring at birth or on day 4 after birth, or the viability index on day 4. There were no external anomalies in any group. The NOEL is considered to be 1000 mg/kg/day for both the reproductive performance of the parents and development of the offspring.
| Purity | : | ca. 97 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA/pKM101 |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98), Sodium azide (TA1535), 9-Aminoacridine hydrochloride (TA1537) and N-Ethyl-N'-nitro-N-nitrosoguanidine (WP2 uvrA/pKM101) +S9 mix; 2-Aminoanthracene (five strains) |
| Dosage | : | -S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (five strains) +S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (five strains) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA/pKM101
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | ca. 97 % |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Vehicle | : | Isotonic sodium chloride solution |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Benzo[a]pyrene |
| Dosage | : | -S9 mix (6 hr short-term treatment); 0, 12.5, 25, 50, 100, 200, 400 μg/mL +S9 mix (6 hr short-term treatment); 0, 100, 200, 400, 800, 1600, 2400 μg/mL -S9 mix (24 hr continuous treatment); 0, 50, 100, 200, 300, 400, 500 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Nihon Bioresearch Inc., 6-104, Majima, Fukuju-cho, Hashima, Gifu, 501-6251, Japan. Tel +81-58-392-6222 Fax +81-58-392-1284 |
| 2) | The test was performed by the Safety Assessment Laboratory, Panapharm Laboratories Co., Ltd., 1285 Kurisaki-machi, Uto-shi, Kumamoto, 869-0425, Japan. Tel +81-964-23-5111 Fax +81-964-23-2282 |
| 3) | The tests were performed by the Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki 314-0255, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |