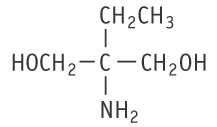
Molecular formula: C5H13NO2 Molecular weight: 119.16
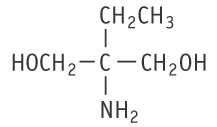
A single dose oral toxicity test of 2-amino-2-ethyl-1,3-propanediol was conducted in female rats at 300 and 2000 mg/kg. No deaths occurred at either dose level, and the lethal dose was estimated to be higher than 2000 mg/kg. No abnormalities were observed regarding the general condition or body weights of any animal or at necropsy.
Oral toxicity was further studied in rats according to the OECD repeated dose Test Guideline 422 at doses of 0, 250, 500 and 1000 mg/kg. Open field examination revealed low values for rearing in males of the 1000 mg/kg group in weeks 3, 4, 5 and 6 of the administration. Histopathological examination revealed cell infiltration in the mucosa of the forestomach and glandular stomach, erosion in the glandular stomach, increased numbers of globular leukocytes in the glandular stomach, thickening of the glandular stomach mucosa and thickening of the limiting ridge in males of the 1000 mg/kg group.
The NOELs for repeated dose oral toxicity test were judged to be 500 mg/kg/day for males and 1000 mg/kg/day for females.
With regard to reproductive/developmental toxicity items, the test substance showed no adverse effects on any relevant parameters.
The NOELs for the reproductive/developmental toxicity test were judged to be 1000 mg/kg/day for both male and female parent animals and also for offspring.
Reverse mutation assays using microorganisms (Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA) were conducted to assess the potential of 2-amino-2-ethyl-1,3-propanediol to induce gene mutations. No mutagenic activity was found in the bacteria under the present experimental conditions.
In vitro chromosomal aberration tests using cultured mammalian cells (CHL/IU) were conducted to assess the potential of 2-amino-2-ethyl-1,3-propanediol to induce chromosomal aberrations. Neither structural nor numerical chromosome aberrations were detected under the present experimental conditions.
| Purity | : | 99.4 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 423 |
| Route | : | Oral(gavage) |
| Doses | : | 300, 2000 mg/kg |
| Numberofanimals/group | : | Females, 6 |
| Vehicle | : | Water for injection |
| GLP | : | Yes |
Test results:
| Purity | : | 99.4 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral(gavage) |
| Dosage | : | 0(vehicle), 250, 500, 1000 mg/kg/day |
| Number of animals/group | : | Males, 17; females, 17(0 and 1000 mg/kg/day) Males, 12; females, 12(250 and 500 mg/kg/day) |
| Vehicle | : | Water for injection |
| Administration period | : |
Males, 42 days |
| Terminal killing | : |
Males, day 43 |
| GLP | : | Yes |
Test results:
No test article-related effects were observed with regard to clinical signs, function tests, grip strength, spontaneous movement, body weights, food consumption, urinalysis (including water intake), hematology, blood chemistry, findings at necropsy or organ weights.
Open field examination revealed low values for rearing in males of the 1000 mg/kg group in weeks 3, 4, 5 and 6 of the administration, but values were normal in the test at the end of the recovery period.
Histopathological examination at the end of the administration period revealed cell infiltration in mucosa of the forestomach and glandular stomach, erosion in the glandular stomach, increased numbers of globular leukocytes in the glandular stomach, thickening of the glandular stomach mucosa and thickening of the limiting ridge in males of the 1000 mg/kg group. At the end of the recovery period, increased numbers of globular leukocytes in the glandular stomach and thickening of the limiting ridge were still observed, but their severity had decreased, suggesting reversibility.
<Reproductive and developmental toxicity>
No test article-related effects were observed in the estrous cycle, number of days until copulation, copulation index, insemination index, or fertilization index. Furthermore, there were no effects of test article administration on the gestation period, birth index, numbers of corpora lutea, numbers of implantations, implantation index, parturition or nursing behavior, numbers of stillborn pups, still birth index, numbers of liveborn pups, liveborn index, sex ratio, observation of liveborn pups at birth or necropsy at 4 days postpartum, as well as body weights or viability.
<Evaluation>
From the above results, the NOELs for repeat dose toxicity of 2-amino-2-ethyl-1,3- propanediol were judged to be 500 mg/kg/day for males and 1000 mg/kg/day for females. The NOELs for reproductive/developmental toxicity were judged to be 1000 mg/kg/day for both male and female parent animals, and also for offspring.
| Purity | : | 99.4 wt% |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Water for injection |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98 and WP2 uvrA), sodium azide (TA1535) and 2-methoxy-6-chloro-9-[3-(2-chloroethyl)- aminopropylamino] acridine-2HCl (TA1537) +S9 mix; Benzo[a]pyrene (TA100, TA98, TA1537) and 2-aminoanthracene (TA1535 and WP2 uvrA) |
| Dosage | : | -S9 mix; 0, 156, 313, 625, 1250, 2500 and 5000 μg/plate (all strains) +S9 mix; 0, 156, 313, 625, 1250, 2500 and 5000 μg/plate (all strains) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| GLP | : | Yes |
Genetic effects :
S. typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| without metabolic activation: | [*] | [ ] | [ ] |
| with metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.4 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Vehicle | : | Physiological saline |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix(short-term treatment); 0, 75, 150, 300, 600 and 1200 μg/mL +S9 mix(short-term treatment); 0, 75, 150, 300, 600 and 1200 μg/mL -S9 mix(continuous treatment 24 hr); 0, 75, 150, 300, 600 and 1200 μg/mL -S9 mix(continuous treatment 48 hr); 0, 75, 150, 300, 600 and 1200 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
No increase in chromosomal aberrations was observed in the test with either the short- term treatment (-S9 and +S9) or continuous treatment.
Genetic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Bozo Research Center Inc., 1284 Kamado, Gotemba-shi, Shizuoka, 412-0039, Japan. Tel & Fax +81-550-82-9922. |