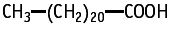
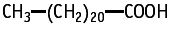
Docosanoic acid was studied for oral toxicity in rats in a single dose toxicity test at doses of 0 and 2000 mg/kg. An LD50 value of more than 2000 mg/kg was found for both sexes.
Docosanoic acid was studied for oral toxicity in rats in an OECD combined repeat dose and reproductive/developmental toxicity screening test at doses of 0, 100, 300 and 1000 mg/kg/day.
With regard to repeat dose toxicity, no deaths or abnormalities in general condition were observed in any male and female animals. Also, there were no adverse changes related to the dosing of the compound in body weights and food consumption. The findings for internal organs and histopathological and biochemical examinations revealed no adverse changes in any animals.
With regard to the reproductive/developmental toxicity, there were no adverse effects on copulation, ovulation and fertility in any docosanoic acid-treated animals. In addition, no adverse changes related to the dosing were observed in gestation length, delivery and lactation. There were no changes in sex ratio, body weights and viability of the docosanoic acid-treated pups compared to those of the control pups. Also, no morphological abnormalities were noted in any pups of the docosanoic acid-treated group.
In conclusion, the NOEL for toxicity of docosanoic acid is considered to be 1000 mg/kg/day in male and female animals, and that for reproductive and developmental toxicity was also 1000 mg/kg/day in males and females.
Docosanoic acid was not mutagenic in Salmonella typhimurium TA100, TA1535, TA98, TA1537 and Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
Docosanoic acid did not induce structural chromosomal aberrations and polyploidy in CHL cells, with or without an exogenous metabolic activation system.
| Purity | : | 85.9 wt% |
| Test species/strain | : | Rat/Crj:CD(SD) |
| Test method | : | OECD Test Guideline 401 |
| Doses | : | 2000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| GLP | : | Yes |
Test results:
LD50: Male, > 2000 mg/kg; female, > 2000 mg/kg
| Purity | : | 85.9 % |
| Test species/strain | : | Rat/Crj:CD(Sprague-Dawley) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/ Developmental Toxicity Screening Test |
| Route | : | Oral(Gavage) |
| Doses | : | 0(vehicle), 100, 300, 1000 mg/kg/day |
| Number of animals/group | : | Males, 13; females, 13 |
| Vehicle | : | Corn oil |
| Administration | : | Males, 42 days Females, from 14 days prior to mating to day 3 of lactation |
| Terminal killing | : | Males, day 43 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
a)Males: No deaths or abnormalities in general condition were observed in any of the treated groups. Also, there were no changes related to the dosing of compound in body weight gain and food consumption. At autopsy following treatment for 42 days, no adverse effects were found for internal organs and findings of histopathological, hematological and biochemical examinations.
b)Females: No deaths were observed in any treated groups. Also, there were no changes related to the dosing of compound in general condition, body weight gain and food consumption. No abnormal findings were evident at autopsy on postpartum day 4 and on histopathological examination.
<Reproductive and developmental toxicity>
The compound showed no adverse effects on copulation or fertility. No changes related to the dosing of compound were observed in gestation length, gestation index, delivery and lactation. The compound did not demonstrate any adverse effects on the sex ratio, body weights or viability of pups. Also, no morphological abnormalities in pups were observed in any of the treated groups.
The no observed effect dose level(NOEL) for toxicity is considered to be 1000 mg/kg/day in males and females, and that for reproductive and developmental toxicity also 1000 mg/kg/day in males and females.
| Purity | : | 85.9 % |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537, E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan)and OECD Guidelines No. 471 and 472 |
| Procedures | : | Pre-incubation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide(TA100, TA98 and WP2 uvrA), Sodium azide(TA1535), 9-Aminoacridine hydrochloride(TA1537) +S9 mix; 2-Aminoanthracene(all strains) |
| Doses | : | -S9 mix; 156, 313, 625, 1250, 2500 and 5000 μg/plate +S9 mix; 156, 313, 625, 1250, 2500 and 5000 μg/plate |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
S. typhimurium TA100, TA1535, TA98 and TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 85.9 % |
| Type of cell used | : | Chinese hamster lung(CHL)cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan)and OECD Guideline No. 473 |
| Vehicle | : | 1% Carboxymethylcellulose sodium |
| Positive controls | : | -S9 mix, Mitomycin C +S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix(24hr continuous exposure): 0, 350, 700, 1400, 2800 μg/mL -S9 mix(48hr continuous exposure): 0, 288, 575, 1150, 2300 μg/mL -S9 mix(short-term exposure): 0, 875, 1750, 3500 μg/mL +S9 mix(short-term exposure): 0, 875, 1750, 3500 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-0025, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), Japan, 582-2 Shioshinden Arahama, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |