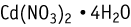
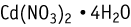
Cadmium nitrate tetrahydrate was studied for oral toxicity in rats in an OECD combined repeat dose and reproductive/developmental toxicity screening test at doses of 0, 1.5, 5, 15, and 50 mg/kg/day (the dose levels are shown as cadmium nitrate anhydrate).
Significantly lower body weights and food consumption were noted in males at 15 and 50 mg/kg/day. Blood examination of the males at the termination of administration revealed significantly lower values for the erythrocyte count, hemoglobin and hematocrit at 15 and 50 mg/kg/day. On blood chemical analysis, AST, ALT, γ-GTP, total protein and albumin were significantly lowered at 50 mg/kg/day, and similar changes at 15 mg/kg/day. Decreases in organ weight were noted in the thymus and increase were marked in the adrenal at 50 mg/kg/day. On histopathological examination, vacuolar degeneration of tubular epithelium in the kidneys at 50 mg/kg/day and hypertrophy of the zona fasciculata the adrenals at 15 and 50 mg/kg were observed. In the testes, focal degeneration of seminiferous tubules were noted at 50 mg/kg.
Five of 12 females in 50 mg/kg/day group died on 19-21 day of gestation. Decrease in locomotor activity, hypothermia, and perioral smudge were apparent in these animals. Lower body weights were noted at 15 and 50 mg/kg/day throughout the pregnancy and lactation periods. On the necropsy at the 4th day after parturition weight of thymus were significantly lower in all the treated groups and the adrenal weights were higher at 15 mg/kg/day. On histopathological examination, cellular infiltration in the submucosa of the forestomach at 1.5 mg/kg/day and higher, squamous hyperplasia in the forestomach at 15 mg/kg/day and higher, and ulceration, erosion and subepithelial edema in the forestomach at 50 mg/kg/day the thymus showed atrophy in all the treated groups; degeneration of tubular epithelium in the kidneys was noted at 15 mg/kg and vacuolar degeneration of tubular epithelium at 50 mg/kg; and the adrenals showed hypertrophy of the zona fasciculata at 1.5 mg/kg and higher.
Thus, the NOELs for repeated dose toxicity are considered to be at less than 1.5 mg/kg/day for both sexes.
No changes attributable to the compound administration were noted in reproductive performances. However, all pups from 2 dams at 15 mg/kg and from 1 dam at 50 mg/kg had died after delivery. One dam at 5 mg/kg showed abnormal nursing behavior, and all her pups died. Three dams at 15 mg/kg also showed abnormal nursing behavior, and all pups from 1 of these died. At 50 mg/kg, 4 dams showed abnormal nursing behavior, and all their pups died.
The NOELs for reproductive performance are considered to be at 50 mg/kg/day for males and at 1.5 mg/kg/day for females.
Regarding the pups, the number of live pups and viability index on Day 4 of lactation were lower in all the treated groups. The body weights of the pups of both sexes were lower in all the treated groups on Days 4 of lactation.
The NOEL for pups is considered to be less than 1.5 mg/kg/day.
Reverse mutation assays using microorganisms (Salmonella typhimurium, Escherichia coli) were conducted to assess the potential of nitric acid, cadmium salt tetrahydrate to induce gene mutations. Cadmium nitrate tetrahydrate did not induce gene mutations in bacteria under the conditions of this study.
In vitro chromosomal aberration tests using cultured cells (CHL/IU) were conducted to assess the potential of nitric acid, cadmium salt tetrahydrate to induce chromosomal aberrations.
Cadmium nitrate tetrahydrate did induce chromosomal aberrations in cultured cells under the conditions of this study.
| Purity | : | 99.07 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 62.5, 125, 250, 500 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Water for injection |
| GLP | : | Yes |
Test results:
The LD50 values (as cadmium nitrate anhydrate) were found to be 243 mg/kg (95 % confidence limits: 142-454 mg/kg) for males and 188 mg/kg (95 % confidence limits: incalculable) for females.
| Purity | : | 99.07 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (vehicle), 1.5, 5, 15, 50 mg/kg |
| Number of animals/group | Males, 12; females, 12 | |
| Vehicle | : | Water for injection |
| Administration period | : | Males, 49 days Females, from 14 days before mating to day 3 of lactation |
| Terminal killing | : | Males, day 50 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
Regarding the males, lowering of body weights and consumption were noted at 15 mg/kg and above. On urinalysis, tendencies for the urinary volume to be higher and for pH to be lower were noted at 50 mg/kg. In the hematological examination, lower values for fibrinogen were noted at 1.5 mg/kg and above, and for hemoglobin, MCV, MCH, PT, and APTT at 15 mg/kg and above, as well as lower values sortie erythrocyte count and hematocrit and a higher value for the platelet count were noted at 50 mg/kg. In the blood chemical analysis, lower values for total protein and higher values for inorganic phosphorus were noted at 5 mg/kg and above, lower values for ALP and higher values for ALT were noted at 15 mg/kg and above, and lower values for albumin and Na and higher values for AST, γ-GTP, and total cholesterol were noted at 50 mg/kg. Organ weight measurement, revealed decrease of the absolute thymus weights, increase of the absolute adrenal weights, and increase of the relative liver, spleen, kidney, and adrenal weights at 50 mg/kg. On histopathological examination, changes were noted as follows: in the lungs, accumulation of foam cells and focal inflammatory cell infiltration at 5 mg/kg and above; in the liver, focal necrosis of hepatocytes at 5 and 50 mg/kg; in the pancreas, focal degeneration of acinar cells at 50 mg/kg; in the kidneys, vacuolar degeneration of tubular epithelium at 50 mg/kg; in the adrenals, hypertrophy of the zona fasciculata at 15 and 50 mg/kg; in the testes, focal degeneration of seminiferous tubules at 50 mg/kg; and in the bone marrow, focal degeneration and decreased hematopoiesis at 50 mg/kg.
Regarding the females, dead animals were noted at 50 mg/kg, along with decrease in locomotor activity, hypothermia, and perioral soiling. Lowering of body weights was noted at 50 mg/kg before initiation of mating and during the mating period and at 15 mg/kg and above during the pregnancy and lactation periods. Food consumption was lowered or tended to be lower at 15 mg/kg and above before initiation of mating and during the pregnancy and lactation periods. Organ weight measurement, revealed changes as follows: the absolute and relative thymus weights were lowered or tended to be lower at 1.5 mg/kg and higher; and the absolute and relative adrenal weights were higher or tended to be higher, the absolute spleen weights was lower or tended to be lower, and the relative liver, kidney, and ovary weights were higher or tended to be higher at 15 mg/kg and above. In the histopathological examination, changes were noted as follows: in the lungs, accumulation of foam cells at 1.5 mg/kg, focal inflammatory cell infiltration at 5 mg/kg, and accumulation of foam cells and focal inflammatory cell infiltration at 15 and 50 mg/kg; in the liver, focal necrosis of hepatocytes at 5 mg/kg and focal necrosis and vacuolization of hepatocytes at 50 mg/kg; in the stomach, cellular infiltration in the submucosa of the forestomach at 1.5 and 5 mg/kg, cellular infiltration in the submucosa of the forestomach, squamous hyperplasia in the forestomach, and cellular infiltration in the submucosa of the forestomach at 15 mg/kg, and cellular infiltration in the submucosa of the forestomach, ulceration in the forestomach, erosion in the forestomach, and subepithelial edema in the forestomach at 50 mg/kg; in the thymus, atrophy at 1.5 mg/kg and above; in the kidneys, degeneration of tubular epithelium at 15 mg/kg and vacuolar degeneration of tubular epithelium and degeneration of tubular epithelium at 50 m/kg; and in the adrenals, hypertrophy of zona fasciculata at 1.5 mg/kg and above.
The NOELs for repeated dose toxicity are considered to be less than 1.5 mg/kg/day for both sexes.
<Reproductive and developmental toxicity>
Regarding reproductive/developmental toxicity, no changes attributable to the compound administration were noted in terms of the number of estrous cases, the copulation index, number of days before copulation, number of pregnant females, or the gestation length. Regarding the delivery conditions, all pups had died from 2 dams at 15 mg/kg and 1 dam at 50 mg/kg. Regarding the nursing conditions, 1 dam at 5 mg/kg showed abnormal lactation behavior, and all her pups died. Three dams at 15 mg/kg also showed abnormal lactation behavior, and all pups from 1 of these died. At 50 mg/kg, 4 dams showed abnormal lactation behavior, and all their pups died. No changes attributable to the compound administration were noted in terms of the fertility index, number of corpora lutea, number of implantation sites, or the implantation rate. The gestation index tended to be lowered at 15 mg/kg and above.
The NOELs for reproductive performance are considered to be at 50 mg/kg/day for males and at 1.5 mg/kg/day for females.
Regarding the pups, tendencies for the numbers of pups on Day 0 of lactation and birth index to be lower, lower values for the live birth index, and higher values for the numbers of stillbirths were noted at 15 mg/kg and above. Regarding general signs, hypothermia was noted at 15 mg/kg and above. The number of live pups on Day 4 of lactation and the viability index on Day 4 of lactation tended to be lower or were lower at 15 mg/kg and above. The body weights of both sexes were lower or tended to be lower at 1.5 mg/kg and above on Days 0 and 4 of lactation.
The NOEL for pups is considered to be at less than 1.5 mg/kg/day.
| Purity | : | 99.07 % |
| Test species/strain | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals(Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Water for injection |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide, (TA100, TA98 and WP2 uvrA), Sodium azide(TA1535), 9-Aminoacridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | -S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate(all strains for dose-finding study) +S9 mix; 0, 2.29, 6.86, 20.6, 61.7, 185, 556, 1667, 5000 μg/plate(all strains for dose-finding study) -S9 mix; 0, 4.88, 9.77, 19.5, 39.1, 78.1, 156, 313 μg/plate(TA100, TA1535, TA1537) -S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (WP2 uvrA) -S9 mix; 0, 19.5, 39.1, 78.1, 156, 313, 625 μg/plate (TA98) +S9 mix; 0, 39.1, 78.1, 156, 313, 625, 1250, 2500 μg/plate(TA100, TA98, TA1537) +S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (TA1535, WP2 uvrA) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.07 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals(Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Water for injection |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 0.375, 0.75, 1.50, 3.00 μg/mL +S9 mix (short-term treatment); 0, 3.00, 6.00, 12.0 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [*] | [ ] | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Nihon Bioresearch Inc., 6-104 Majima, Fukuju-cho, Hashima, Gifu, 501-6251, Japan. Tel +81-58-392-6222 Fax +81-58-392-1284 |
| 2) | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), 582-2 Shioshinden, Fukude-cho, Iwata-gun, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |