

|
|
Printing Date
|
2017-10-30 11:34:25 JST
|
|
Restriction of specific regulatory purposes
|
|
|
Confidentiality
|
|
|
Name
|
2,3,4,4'-Tetrahydroxybenzophenone
|
|
Legal entity owner
|
National Institute of Health Sciences / Tokyo / Japan
|
|
UUID
|
IUC5-240cac4f-f27d-4ed7-ab4d-9fde7ed35f68
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2016-12-21 15:06:08 JST
|
|
|
Remarks
|
|
Chemical name
|
2,3,4,4'-Tetrahydroxybenzophenone
|
|
Legal entity
|
|
|||||||||||||
|
UUID
|
IUC5-81f8cc24-0026-4622-bd2a-06850fde77f0
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 16:02:42 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
OECD Test Guideline study under GLP condition
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
publication
|
MHLW
|
2006
|
Single Dose Oral Toxicity Test of 2,3,4,4'-Tetrahydroxybenzophenone in Rats
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
BoZo Research Center
|
|
data published
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 423 (Acute Oral toxicity - Acute Toxic Class Method)
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
31127-54-5
|
|
- Name of test material (as cited in study report): 2,3,4,4'-Tetrahydroxybenzophenone
- Analytical purity: 99.86% - Lot/batch No.: GL01 - Stability under test conditions: The stability of test material was identified by analysis of the remainder. - Storage condition of test material: At a cold place (temperature 2~8℃) in a light resistant container |
|
rat
|
|
other: Crl:CD(SD)
|
|
female
|
|
TEST ANIMALS
- Source :Charles River Japan Inc. - Age at study initiation: 6 weeks old - Weight at study initiation: Females, 123-135 g - Fasting period before study: Approximately 16 hrs - Housing:1/cage - Diet (e.g. ad libitum): Ad libitum except fasting period for 16 hrs before administration to 6 hrs after administration - Water (e.g. ad libitum):Ad libitum - Acclimation period:7 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 23±3 ℃(actual temperature: 21-25℃) - Humidity (%):50 ± 20% (actual humidity: 40-59%) - Air changes (per hr): Approximately 10-15 times/hr - Photoperiod (hrs dark / hrs light):12 hrs light / 12 hrs dark |
|
oral: gavage
|
|
corn oil
|
|
- Amount of vehicle (if gavage):10 ml/kg bw
|
|
2000 mg/kg bw
|
|
3 (1st step group) and 3 (2nd step group)
|
|
no
|
|
- Duration of observation period following administration: 14 days
- Frequency of observations: nearly successive observation (from time just to 1 hr after administration) and observation of every 2 hr (from 2 hr – 6 hr after administration) (day 0); once a day (from day 1-day14) - Frequency of weighing : just before administration (day 0), and 1,3,7 and 14 day after administration - Necropsy of survivors performed: yes |
|
Sex
|
Endpoint
|
Effect level
|
Based on
|
95% CL
|
Remarks
|
|
female
|
LD50
|
> 2000 mg/kg bw
|
|
No deaths were observed in any group.
|
|
No changes related to the test substance were observed in any group.
|
|
No changes related to the test substance were observed in any group.
|
|
No changes related to the test substance were observed in any group.
|
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF31127 -54 -5a.pdf |
The acute oral median lethal dose (LD50) for 2,3,4,4'-tetrahydroxybenzophenone was established at > 2,000 mg/kg bw in female rats on the basis of a study conducted according to the Organisation for Economic Co-operation and Development Test Guideline (OECD TG) 423. The substance caused no deaths or clinical signs of toxicity at 2,000 mg/kg bw. |
|
UUID
|
IUC5-b49d0715-b5a9-4ebc-bed0-1f609412fd9f
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 16:04:12 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
GLP guideline study
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW Japan
|
2009
|
Combined repeat dose and reproductive/developmental toxicity screening test of 2,3,4,4'-Tetrahydroxybenzophenone by oral administration in rats
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
BoZo Research Center
|
|
data published
|
|
7.8.1 Reproductive/developmental toxicity.001
|
|
combined repeated dose and reproduction / developmental screening
|
|
no
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 422 (Combined Repeated Dose Toxicity Study with the Reproduction / Developmental Toxicity Screening Test)
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
31127-54-5
|
|
- Name of test material (as cited in study report): 2,3,4,4'-Tetrahydroxybenzophenone
- Purity: 99.91% - Impurities (identity and concentrations): Unknown - Lot/batch No.: JSCXB - Stability under test conditions: Stable - Storage condition of test material: Refrigeration - Dosing solution storage condition: Room temperature and protected from light - Other: The dosing solution was used within 7 days of preparation. |
|
rat
|
|
other: Crl: CD(SD)
|
|
male/female
|
|
TEST ANIMALS
- Source: Charles River Laboratories Japan, Inc. Atsugi - Age at study initiation: 10 weeks - Weight at study initiation: Males: 335-391 g; Females: 202-249 g - Housing: Steel wire-mesh cage (250 mm x 350 mm x 200 mm ) - Diet: ad libitum - Water: ad libitum - Acclimation period: 14 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 21-23 - Humidity (%): 46-61 - Air changes: 10-15 times / hr - Photoperiod: 12 hrs dark / 12 hrs light |
|
oral: gavage
|
|
corn oil
|
|
PREPARATION OF DOSING SOLUTIONS:
VEHICLE - Amount of vehicle (if gavage): 5 mL/kg bw - Lot/batch no. (if required): SDE2487 |
|
(P) Males: 42 days including 14 days pre-mating and mating periods, and thereafter 14 days (P) Females: 42-55 days including 14 days pre-mating, mating and gestation periods, and the days until day 4 of lactation; satellite animals: 42 days.
|
|
Once/day, 7days/week
|
|
0 (vehicle), 100, 300, and 1000 mg/kg bw/day
|
|
|
Basis
|
actual ingested
|
|
12 animals/sex/dose as a main dose group,
5 males and 5 females at 0 and 1000 mg/kg bw/day as a satellite group (without mating) |
|
yes, concurrent vehicle
|
|
- Dose selection rationale: Doses in this test were set based on the results of the following study: 14-day repeated dose oral toxicity test (doses: 100, 300, and 1000 mg/kg bw/day). In the 14-day repeated dose oral toxicity test, abnormalities were observed in animals in the 1000 mg/kg bw/day group, such as low values of body weight and food consumption an increase in liver weight. No effects were observed at 300 mg/kg bw/day. On the basis of these effects, a dose level of 1000 mg/kg was selected as the maximum dose expecting to induce the toxic changes, and then dose levels of 300 and 100 mg/kg bw/day were selected as a middle dose and a minimum dose levels, respectively, in accordance with a common ratio of approximately 3.
- Rationale for animal assignment (if not random): Body weight-balanced randomization - Post-exposure recovery period in satellite groups: 14 days |
|
CAGE SIDE OBSERVATIONS: Yes
- Time schedule: Males and females: once before the start of administration, 3 times/day during the administration period, and once during the recovery period DETAILED CLINICAL OBSERVATIONS: Yes The functional observational battery testing (FOB) was performed on all animals. Among the measures in the FOB, detailed clinical observations were made before the initiation of dosing. Thereafter, in males of the main groups, detailed clinical observations were made once a week. Also in females of the main groups, detailed clinical observations were made once a week in pre-mating and mating periods thereafter, and then those were made on days 1,7,14 and 20 of gestation, and on day 4 of lactation. For the satellite group, detailed clinical observations were made once a week in dosing and recovery periods. Sensory motor reflexes, forelimb and hindlimb grip strengths, and motor activity were measured on week 6 of administration period (main/recovery group animals) and week 2 of recovery period (recovery group animals). BODY WEIGHT: Yes - Time schedule for examinations: Males (main) & males and females (recovery group): Days 1, 4, 8, 11, 15, 22, 25, 29, 32, 36, 39, 42, and the day of necropsy (after ca. 16h-fasting) in dosing period Males and females (recovery group): Days 1, 4, 8, 11, 14, and the day of necropsy (after ca. 16h-fasting) in recovery period Females (main group): Twice a week during the precopulation period (days 1, 4, 8, 11, and 15); gestation days 0, 4, 7, 11, 14, 17, and 20; lactation days 0 and 4; and the day of necropsy (after ca. 16 h-fasting) FOOD CONSUMPTION AND COMPOUND INTAKE (if feeding study): - Food consumption: Yes Males (main) & males and females (recovery group): Days 1, 4, 8, 11, 15, 32, 36, and 39 in dosing period Males and females (recovery group): Days 1, 4, 8, 11, and 14 in recovery period Females (main group): Days 1, 4, 8, 11, and 15; gestation days 1, 4, 7, 11, 14, 17, and 20; lactation days 2 and 4 OPHTHALMOSCOPIC EXAMINATION: No HAEMATOLOGY: Yes - Time schedule for collection of blood: Blood was collected on the day of necropsy - Anaesthetic used for blood collection: Yes (ether) - Animals fasted: Yes, 16-20h - How many animals: 5 sex/dose/group - Parameters checked in table were examined. CLINICAL CHEMISTRY: Yes - Time schedule for collection of blood: Same as hematology - Animals fasted: Same as hematology - How many animals: Same as hematology - Parameters checked in table were examined. URINALYSIS: Yes (males only) - Time schedule for collection of urine: Day 36-37 in dosing period, day 8-9 in recovery period - Metabolism cages used for collection of urine: No data - Animals fasted: fasting and only water at libitum (4h-urine), no fasting (20h-urine) |
|
GROSS PATHOLOGY: Yes, whole organs and tissues (see tables)
HISTOPATHOLOGY: Yes (see tables) |
|
Organ weight: Brian, thyroids (including parathyroids), thymus, heart, liver, spleen, kidneys, adrenals, testes, epididymis
|
|
The data were analyzed for homogeneity of variance by the Bartlett test. If variances were homogeneous, data was analyzed by the Dunnett test, whereas heterogeneous data was analyzed by the Dunnett type mean rank test (p<0.05, two-sided).
In the recovery test, these values of two groups were analyzed by F test. If variances were homogeneous, data was analyzed by the Student t-test, whereas heterogeneous data was analyzed by the Aspin-Welch t-test (p<0.05, two-sided). |
|
Endpoint
|
Effect level
|
Based on
|
Sex
|
Basis for effect level / Remarks
|
|
LOAEL
|
100 mg/kg bw/day (actual dose received)
|
test mat.
|
male/female
|
effects on the cecum
|
|
yes
|
|
yes
|
|
yes
|
|
not examined
|
|
not examined
|
|
not examined
|
|
yes
|
|
yes
|
|
yes
|
|
yes (see clinical signs.)
|
|
yes
|
|
yes
|
|
yes
|
|
not examined
|
|
CLINICAL SIGNS AND MORTALITY
Males: No dead or moribund animals were observed. Salivation was observed at 1000 mg/kg bw/day. Females: After delivery (day 0 of lactation), one animal died at 1000 mg/kg bw/day. Home cage observation: No effects. In-the-hand observation: No effects. Open field observation: No effects. -Sensory motor reflexes: Auditory response, approach response, touch response, tail pinch response, pupillary reflex, aerial righting reflex: No effects. Landing foot splay: High value in females at 1000 mg/kg bw/day (main group, day 4 of lactation) -Forelimb and hindlimb grip strengths: Low value of hindlimb grip strength in males at 1000 mg/kg bw/day in the 6-week of dosing -Motor activity: High value in females at 300 mg/kg bw/day (main group, day 4 of lactation) without dose-response relationship. In females, high value at 1000 mg/kg bw/day in the 6-week of dosing in the recovery group, but not in the main group at the same dose. (No toxicological effects) BODY WEIGHT AND WEIGHT GAIN Males: Low values of body weight gain was observed at 1000 mg/kg bw/day in main group. Females: Low value of body weight gain was observed at 1000 mg/kg bw/day at the ends of pre-mating and gestation periods. Low value of body weight was observed at 300 mg/kg bw/day on day 4 of lactation. At the end of recovery period, high value of body weight gain was observed in both sexes at 1000 mg/kg bw/day. FOOD CONSUMPTION Males: Low value of food consumption was observed at 1000 mg/kg bw/day on day 4 of dosing, and significant increase was observed on days 36-42. Females: Low value of food consumption was observed at 1000 mg/kg bw/day on day 4 of dosing, high value on day 15 of dosing, and then low value on day 20 of gestation and day 2 of lactation. Low value of food consumption was observed at 300 mg/kg bw/day on day 4 of dosing and day 2 of lactation. HAEMATOLOGY Low values of RBC, Hb, Ht and MCHC, and high values of platelet, neutrophilic count and monocyte count were observed at 1000 mg/kg bw/day at the end of dosing. At the end of recovery period, low values of RBC and Hb, and high value of reticulocyte were observed in males at 1000 mg/kg bw/day. Other significant findings in the table were within the normal ranges of physiological variability. CLINICAL CHEMISTRY High value of inorganic phosphorus was observed at 300 mg/kg bw/day and more at the end of dosing. Other significant findings in the table were within the normal ranges of physiological variability. URINALYSIS (only males; statistical analysis was not performed on qualitative items.) Occult blood was observed in all administered males, and the degree of occult blood was enhanced in a dose-dependent manner. Dark yarrow color was observed in each 2-3 males at 100 mg/kg bw/day and more. ORGAN WEIGHTS Low values of absolute and relative thymus weights were observed in females at 300 mg/kg bw/day and more at the end of dosing. High value of relative liver weight was observed in both sexes at 1000 mg/kg bw/day at the end of dosing, and in females at the end of recovery period. Other significant findings in the table were within the normal ranges of physiological variability. GROSS PATHOLOGY In the dead female at 1000 mg/kg bw/day, small sizes of spleen and thymus were observed. Undernourishment of general descriptions was observed in one female at 1000 mg/kg bw/day. Dark discoloration of liver was observed in six males at 1000 mg/kg bw/day. Small size of thymus was observed in one female at 300 mg/kg bw/day, and in three females at 1000 mg/kg bw/day. Other findings in the table were considered to be incidental due to low frequency of appearance and/or pathological properties. HISTOPATHOLOGY: NON-NEOPLASTIC In the dead female at 1000 mg/kg bw/day, atrophy of white pup in the spleen and atrophy of thymus were observed. [At the end of dosing] Cecum: Single cell necrosis of mucosal epithelial cells was observed in 4 males and 2 females at 100 mg/kg bw/day, in 3 males and 3 females at 300 mg/kg bw/day, and in 8 males and 7 females at 1000 mg/kg bw/day. Diffuse hyperplasia of mucosa was observed in 1 male and 1 female at 100 mg/kg bw/day, in 3 males and 4 females at 300 mg/kg bw/day, and in 7 males and 6 females at 1000 mg/kg bw/day. Liver: Vacuolation of peripheral hepatocytes was dose-dependently decreased in males and females at 300 mg/kg bw/day and more. Thymus: Atrophy was dose-dependently increased in females at 300 mg/kg bw/day and more. [At the end of recovery period] Cecum: Diffuse hyperplasia of mucosa was observed in one male at 1000 mg/kg bw/day. Other findings in the tables were considered to be incidental due to low frequency of appearance and/or pathological properties. |
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF31127 -54 -5d.pdf |
|
Based on the effects on the cecum, the low observed adverse effect level (LOAEL) for repeated oral dosing was determined to be 100 mg/kg bw/day in male and female rats.
|
A combined repeated oral dose toxicity study with the reproduction/developmental toxicity screening test was performed according to OECD TG 422. Male and female rats (12 animals/sex/dose) were administered 2,3,4,4'-tetrahydroxybenzophenone at 0, 100, 300, and 1,000 mg/kg bw/day. Males were dosed for 42 days, including a 14-day pre-mating and mating periods; females were dosed for 41–45 days, including a 14-day pre-mating, mating, and gestation periods and the time until day 4 of lactation. In addition, male and female rats (five animals/sex/dose) were administered 0 and 1,000 mg/kg bw/day for 42 days without mating and examined after a 14-day recovery period. At 1,000 mg/kg bw/day, one female died on day 0 of lactation, salivation was observed in males and a decreased body weight gain was observed in both sexes. Regarding hematology parameters, anemia was observed at the same dose in males. Clinical chemistry studies demonstrated increased inorganic phosphorus at 300 mg/kg bw/day and higher in males. In the thymus, decreased organ weight and atrophy were observed at 300 mg/kg bw/day and higher in females. In the cecum, single cell necrosis of mucosal epithelial cells and diffuse mucosal hyperplasia were observed at 100 mg/kg bw/day and higher in both sexes. In the liver, in both sexes, increased organ weight at 1,000 mg/kg bw/day and decreased vacuolation of the perilobular hepatocytes in a dose-dependent manner at 300 mg/kg bw/day and higher was observed.These changes tended to resolve after the recovery period. On thebasis of the findings in the cecum, the LOAEL for repeated-dose toxicity of 2,3,4,4'-tetrahydroxybenzophenone was determined to be 100 mg/kg bw/day in male and female rats. |
|
UUID
|
IUC5-9c9461e4-d1ec-4fcd-aa97-faf2ecde802a
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2016-12-21 14:50:21 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
OECD Test Guideline study under GLP condition
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW, Japan
|
2006
|
Reverse Mutation Test of 2,3,4,4'-Tetrahydroxybenzophenone on Bacteria.
|
Japan Existing Chemical Data Base (JECDB)
|
Research Institute for Animal Science in Biochemistry & Toxicology (RIAS)
|
|
data published
|
|
gene mutation
|
|
bacterial reverse mutation assay (e.g. Ames test)
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
JAPAN: Guidelines for Screening Mutagenicity Testing Of Chemicals
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
31127-54-5
|
|
- Name of test material (as cited in study report): 2,3,4,4'-Tetrahydroxybenzophenone
- Purity: 100% - Lot/batch No.: GL01 - Storage condition of test material: Refrigeration - Stability under test conditions: The stability of test material was identified by analysis of the remainder. |
|
Species/strain
|
S. typhimurium TA 1535, TA 1537, TA 98 and TA 100
|
|
Metabolic activation
|
with and without
|
|
Metabolic activation system
|
rat liver, induced by phenobarbital and 5,6-benzoflavone
|
|
Species/strain
|
E. coli WP2 uvr A
|
|
Metabolic activation
|
with and without
|
|
Metabolic activation system
|
rat liver, induced by phenobarbital and 5,6-benzoflavone
|
|
-S9 mix: 1.56, 3.13, 6.25, 12.5, 25, 50 μg/plate (TA1537, TA98 strains),
62.5, 125, 250, 500, 1000, 2000 μg/plate (WP2uvrA strain), and 31.3, 62.5, 125, 250, 500, 1000 μg/plate (TA100, TA1535 strains) +S9 mix: 15.6, 31.3, 62.5, 125, 250, 500, 1000 μg/plate (all strains) |
|
- Vehicle(s)/solvent(s) used: DMSO
|
|
Negative controls
|
no
|
|
|
Solvent / vehicle controls
|
yes
|
|
|
True negative controls
|
no
|
|
|
Positive controls
|
yes
|
|
|
Positive control substance
|
|
|
RANGE-FINDING/SCREENING STUDIES:Concentration: 20-5000 μg/plate
Cytotoxic conc.: [-S9mix] Yes; >50 μg/plate (TA 98, TA1537), >1000 μg/plate (TA100, TA1535), >2000 μg/plate (WP2uvrA), [+S9mix] No. METHOD OF APPLICATION: Preincubation DURATION - Preincubation period: 20 min at 37 ℃ - Exposure duration:48 hrs NUMBER OF PLATES: 3 NUMBER OF REPLICATIONS: 2 DETERMINATION OF CYTOTOXICITY - Method: other: growth inhibition |
|
In any strain(s) tested with or without S9 mix, when the mean number of revertant colonies per plate increased twice more than that of the negative control and when the increase was shown to be dose-related and reproducible, the chemical was judged mutagenic.
|
|
No.
|
|
Species/strain
|
S. typhimurium TA 1535, TA 1537, TA 98 and TA 100
|
|
Metabolic activation
|
with and without
|
|
Test system
|
all strains/cell types tested
|
|
Genotoxicity
|
negative
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
Species/strain
|
E. coli WP2 uvr A
|
|
Metabolic activation
|
with and without
|
|
Test system
|
all strains/cell types tested
|
|
Genotoxicity
|
negative
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
|
negative
|
In a bacterial reverse mutation assay using Salmonella typhimurium TA100, TA1535, TA98, and TA1537 and Escherichia coli WP2uvrA (similar to OECD TG 471), 2,3,4,4'-tetrahydroxybenzophenone was negative with or without metabolic activation. |
|
UUID
|
IUC5-22ba9be0-7295-4c58-9fdd-5b2112f052bb
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 16:04:52 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
OECD Test Guideline study under GLP condition
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW, Japan
|
2007
|
In Vitro Chromosomal Aberration Test of 2,3,4,4'-Tetrahydroxybenzophenone on Cultured Chinese Hamster Cells.
|
Japan Existing Chemical Data Base (JECDB)
|
BoZo Research Center
|
|
data published
|
|
chromosome aberration
|
|
in vitro mammalian chromosome aberration test
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 473 (In vitro Mammalian Chromosome Aberration Test)
|
no
|
|
according to
|
JAPAN: Guidelines for Screening Mutagenicity Testing Of Chemicals
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
31127-54-5
|
|
- Name of test material (as cited in study report): 2,3,4,4'-Tetrahydroxybenzophenone
- Analytical purity: 99.86% (GL01), 99.91% (JSCXB) - Supplier: Tokyo Chemical Industry Co., Ltd - Lot/batch No.: GL01 and JSCXB - Storage condition of test material: cool and dark place |
|
Chromosome
|
|
Species/strain
|
other: Chinese hamster lung(CHL/IU) cells
|
|
Metabolic activation
|
with and without
|
|
Metabolic activation system
|
rat liver, induced by phenobarbital and 5,6-benzoflavone
|
|
-S9 mix (short-term treatment): 0, 19.5, 39.1, 78.1, 156, 313 ug/mL
+S9 mix (short-term treatment): 0, 39.1, 78.1, 156, 313, 625 ug/mL -S9 mix (continuous treatment, 24 h): 0, 19.5, 39.1, 78.1, 156, 313 ug/mL -S9 mix (continuous treatment, 48 h): 0, 2.44, 4.88, 9.77, 19.5, 39.1 ug/mL [Confirmation test] -S9 mix (short-term treatment): 0, 6.58, 9.88, 14.8, 22.2, 33.3, 50 ug/mL +S9 mix (short-term treatment): 0, 205, 256, 320, 400, 500 ug/mL |
|
- Vehicle(s)/solvent(s) used:DMSO
|
|
Negative controls
|
no
|
||
|
Solvent / vehicle controls
|
yes
|
||
|
True negative controls
|
no
|
||
|
Positive controls
|
yes
|
||
|
Positive control substance
|
|
|
METHOD OF APPLICATION: Exposure duration: [continuous treatment]: 24 hrs [short-term treatment]:6 hrs + 18 hr
SPINDLE INHIBITOR: Colcemid NUMBER OF REPLICATIONS: 2 NUMBER OF CELLS EVALUATED: 200 cells / dose DETERMINATION OF CYTOTOXICITY - Method: relative total growth |
|
For the evaluation of the frequencies of structural aberrations and of polyploidy induced, the following criteria were employed.
Appearance incidence of cells with chromosomal aberrations: Negative (-): < 5%; equivocal (±): 5-10%; positive (+): > 10%. Finally, the substance is positive when the incidence is considered to be dose-related and reproducible. |
|
not used.
|
|
Species/strain
|
other: Chinese hamster lung (CHL/IU) cells
|
|
Metabolic activation
|
with
|
|
Genotoxicity
|
positive (weakly)
|
|
Cytotoxicity
|
yes (625 ug/mL)
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
Species/strain
|
other: Chinese hamster lung (CHL/IU) cells
|
|
Metabolic activation
|
without
|
|
Genotoxicity
|
negative
|
|
Cytotoxicity
|
yes 313 ug/mL (short), 78.1 ug/mL and more (continuous)
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF31127 -54 -5f.pdf |
Anin vitrochromosomal aberration test using CHL/IU cells (OECD TG 473) showed positive. |
|
UUID
|
IUC5-9f1cf02c-892b-4385-87b1-b9308a1b4173
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 16:06:01 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
GLP guideline study
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW Japan
|
Micronucleous test of 2,3,4,4'-Tetrahydroxybenzophenone on rat
|
data unpublished
|
Chemicals Evaluation and Research Institute, Japan
|
|
data published
|
|
chromosome aberration
|
|
micronucleus assay
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
other guideline: Japan (Iyakushin No.1604): Genetic toxicity test guideline of drugs
|
no data
|
|
according to
|
OECD Guideline 474 (Mammalian Erythrocyte Micronucleus Test)
|
no data
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
31127-54-5
|
|
- Name of test material (as cited in study report): 2,3,4,4'-Tetrahydroxybenzophenone
- Analytical purity:99.9% - Lot/batch No.: EPF0296 - Stability under test conditions: stable - Storage condition of test material: room temperature (17.6-20.8°C) in an airtight container. |
|
mouse
|
|
other: CRLj:CD1(ICR)SPF
|
|
male
|
|
TEST ANIMALS
- Source: Charles River Laboratories Japan, Inc. Hino Farm - Age at study initiation: 7 weeks old - Weight at study initiation: 29.6-34.4 g (avg. 31.9 g) - Assigned to test groups randomly: yes - Housing: polycarbonate cag (225 mm × 338 mm × 140 mm) - Diet: ad libitum - Water: ad libitum - Acclimation period: 6 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 22.8-24.1 - Humidity (%):52.1-61.5 - Air changes: 10-15/h - Photoperiod: 12 h dark/ 12 h light (light time: 7:00 to 19:00) |
|
oral: gavage
|
|
- Vehicle(s)/solvent(s) used: corn oil
- Lot/batch no. (if required): 6G2122 |
|
PREPARATION OF DOSING SOLUTIONS:
Dosing solutions were prepared on the day of administration by dissolving the test substance in corn oil. |
|
Twice, 24 h interval
|
|
0 (vehicle), 125, 250, 500, 1000, 2000 mg/kg bw/day
|
|
|
Basis
|
actual ingested
|
|
6 animals/sex/dose
|
|
yes, concurrent vehicle
|
|
mitomycin C
- Route of administration: single intraperitoneal injection - Doses / concentrations: 2 mg/kg/day |
|
Polychromatic erythrocytes from the femur bone marrow
|
|
TREATMENT AND SAMPLING TIMES (in addition to information in specific fields): Cells for specimen were collected 24 h after the last administration.
DETAILS OF SLIDE PREPARATION: Cell suspensions were expanded on the slides, fixed with methanol, and stained with Giemsa. METHOD OF ANALYSIS: microscopy, blind method |
|
Criterion for determining a positive result: A dose-related increase in the number of micronucleated cells.
|
|
The number of micronucleated polychromatic erythrocytes was determined by the Kastenbaum and Bowman method, and Cochran Armitage test;
Ratio of polychromatic erythrocytes to whole erythrocytes by F-test, and t-test. |
|
Sex
|
male
|
|
Genotoxicity
|
negative
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
|
negative
|
|
The test substance did not produce micronuclei in the immature erythrocytes of the test species.
|
Anin vivomicronucleus study (OECD TG 474) showed negative up to the limit dose (2,000 mg/kg bw/day for 2 days) in mice. |
|
UUID
|
IUC5-bae76ab8-3928-4002-9f8e-b2f2b22173cc
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 16:07:17 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
OECD Test Guideline study under GLP condition
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW, Japan
|
2009
|
Combined repeat dose and reproductive/developmental toxicity screening test of 2,3,4,4'-Tetrahydroxybenzophenone by oral administration in rats
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
BoZo Research Center
|
|
data published
|
|
7.5.1 Repeated dose toxicity: oral.001
|
|
screening
|
|
no
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 422 (Combined Repeated Dose Toxicity Study with the Reproduction / Developmental Toxicity Screening Test)
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
31127-54-5
|
|
- Name of test material (as cited in study report): 2,3,4,4'-Tetrahydroxybenzophenone
- Purity: 99.91% - Impurities (identity and concentrations): Unknown - Lot/batch No.: JSCXB - Stability under test conditions: Stable - Storage condition of test material: Refrigeration - Dosing solution storage condition: Room temperature and protected from light - Other: The dosing solution was used within 7 days of preparation. |
|
rat
|
|
other: Crl:CD(SD)
|
|
male/female
|
|
TEST ANIMALS
- Source: Charles River Laboratories Japan, Inc. Atsugi - Age at study initiation: 10 weeks - Weight at study initiation: Males: 335-391 g; Females: 202-249 g - Housing: Steel wire-mesh cage (250 mm x 350 mm x 200 mm ) - Diet: ad libitum - Water: ad libitum - Acclimation period: 14 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 21-23 - Humidity (%): 46-61 - Air changes: 10-15 times / hr - Photoperiod: 12 hrs dark / 12 hrs light |
|
oral: gavage
|
|
corn oil
|
|
PREPARATION OF DOSING SOLUTIONS:
VEHICLE - Amount of vehicle (if gavage): 5 mL/kg bw - Lot/batch no. (if required): SDE2487 |
|
- M/F ratio per cage:1:1
- Length of cohabitation:up to 14 days - Proof of pregnancy: [vaginal plug / sperm in vaginal smear] referred to as [day 0] of pregnancy |
|
yes
|
|
Test suspensions at each concentration to be used for males in week 1 and six week of administration were analyzed by the HPLC method at Bozo Research Center Inc. Results showed that the concentration of the test article in each suspension was 95.7 to 104.5% of the nominal concentration and both values were within the acceptable range (concentration: percentage of the nominal concentration, 100 ± 10%; C.V.: 10% or below)
|
|
(P) Males: 42 days including 14 days pre-mating and mating periods, and thereafter 14 days
(P) Females: 42-55 days including 14 days pre-mating, mating and gestation periods, and the days until day 4 of lactation; satellite animals: 42 days. |
|
Once/day, 7days/week
|
|
0 (vehicle), 100, 300, and 1000 mg/kg bw/day
|
|
|
Basis
|
actual ingested
|
|
12 animals/sex/dose (main dose group), 5 males and 5 females at 0 and 1000 mg/kg bw/day as a satellite group (without mating).
|
|
yes, concurrent vehicle
|
|
CAGE SIDE OBSERVATIONS: Yes
- Time schedule: Males and females: once before the start of administration, 3 times/day during the administration period, and once during the recovery period DETAILED CLINICAL OBSERVATIONS: Yes The functional observational battery testing (FOB) was performed on all animals. Among the measures in the FOB, detailed clinical observations were made before the initiation of dosing. Thereafter, in males of the main groups, detailed clinical observations were made once a week. Also in females of the main groups, detailed clinical observations were made once a week in pre-mating and mating periods thereafter, and then those were made on days 1,7,14 and 20 of gestation, and on day 4 of lactation. For the satellite group, detailed clinical observations were made once a week in dosing and recovery periods. Sensory motor reflexes, forelimb and hindlimb grip strengths, and motor activity were measured on week 6 of administration period (main/recovery group animals) and week 2 of recovery period (recovery group animals). BODY WEIGHT: Yes - Time schedule for examinations: Males (main/recovery group): Days 1, 4, 8, 11, 15, 22, 25, 29, 32, 36, 39, 42, and the day of necropsy (after ca. 16h-fasting) in dosing period Males and females (recovery group): Days 1, 4, 8, 11, 14, and the day of necropsy (after ca. 16h-fasting) in recovery period Females (main group): Twice a week during the precopulation period (days 1, 4, 8, 11, and 15); gestation days 0, 4, 7, 11, 14, 17, and 20; lactation days 0 and 4; and the day of necropsy (after ca. 16 h-fasting) FOOD CONSUMPTION AND COMPOUND INTAKE (if feeding study): - Food consumption: Yes Males (main/recovery group): Days 1, 4, 8, 11, 15, 32, 36, and 39 in dosing period Males and females (recovery group): Days 1, 4, 8, 11, and 14 in recovery period Females (main group): Days 1, 4, 8, 11, and 15; gestation days 1, 4, 7, 11, 14, 17, and 20; lactation days 2 and 4 OPHTHALMOSCOPIC EXAMINATION: No HAEMATOLOGY: Yes - Time schedule for collection of blood: Blood was collected on the day of necropsy - Anaesthetic used for blood collection: Yes (ether) - Animals fasted: Yes, 16-20h - How many animals: 5 sex/dose/group - Parameters checked in table were examined. CLINICAL CHEMISTRY: Yes - Time schedule for collection of blood: Same as hematology - Animals fasted: Same as hematology - How many animals: Same as hematology - Parameters checked in table were examined. URINALYSIS: Yes (males only) - Time schedule for collection of urine: Day 36-37 in dosing period, day 8-9 in recovery period - Metabolism cages used for collection of urine: No data - Animals fasted: fasting and only water at libitum (4h-urine), no fasting (20h-urine) NEUROBEHAVIOURAL EXAMINATION: No |
|
Vaginal smears were collected from all females in the main groups and microscopically examined every day from the day after the start of administration until the day copulation was confirmed. During the pre-mating administration period, vaginal smear pictures were classified as proestrus, estrus, metestrus or diestrus and examined for the frequency of estrus and interval between estruses (estrous cycle). During the mating period, vaginal smears were examined for the presence of sperm.
|
|
Parameters examined in P male parental generations: testes weight, epididymis weight
|
|
PARAMETERS EXAMINED:The following parameters were examined in F1 offspring: Number and sex of pups, stillbirths, live births, postnatal mortality, presence of gross anomalies, and weight gain.
GROSS EXAMINATION OF DEAD PUPS: Yes, for external and internal abnormalities. |
|
SACRIFICE:
Male animals: Rats were euthanized by exsanguination under ether anesthesia on the day after the last administration. Maternal animals: Rats were euthanized by exsanguination under ether anesthesia on day 4 of lactation. GROSS PATHOLOGY, Yes: whole organs and tissues ORGAN WEIGHTS, Yes: Brain, thyroids(including parathyroids), thymus, heart, liver, spleen, kidneys, adrenals, testes, epididymis HISTOPATHOLOGY, Yes: Cerebrum, cerebellum, pituitary gland, spinal cord (thoracic), sciatic nerve, thyroid, parathyroid, adrenal glands, thymus, spleen, submandibular lymph nodes, mesenteric lymph nodes, heart, lung (including the bronchi), stomach, duodenum, jejunum, ileum, cecum, colon, rectum, liver, kidney, bladder, testis, epididymis, ovary, uterus, seminal vesicles, sternum and femur (including bone marrows), macroscopic lesions. |
|
GROSS NECROPSY
- Gross necropsy consisted of external and internal examinations including the cervical, thoracic, and abdominal viscera. |
|
The data were analyzed for homogeneity of variance by the Bartlett test. If variances were homogeneous, data was analyzed by the Dunnett test, whereas heterogeneous data was analyzed by the Dunnett type mean rank test (p<0.05, two-sided).
In the recovery test, these values of two groups were analyzed by F test. If variances were homogeneous, data was analyzed by the Student t-test, whereas heterogeneous data was analyzed by the Aspin-Welch t-test (p<0.05, two-sided). |
|
Each parameter was determined by the following equations:
Copulation index (%) = (No. of copulated animals/No. of co-housed animals) × 100 Fertility index (%) = (No. of pregnant females/No. of copulated females) × 100 Insemination index (%) = (No. of pregnant females/No. of copulated males) × 100 Duration of gestation (days) = day 0 of lactation – day 0 of gestation Delivery index (%) = (No. of females delivered liveborn pups/No. of pregnant females) × 100 Implantation index (%) = (No. of implantation sites/No. of corpora lutea) × 100 Stillborn index (%) = (No. of stillborn pups/Total No. of pups born) × 100 Liveborn index (%) = (No. of liveborn pups/Total No. of pups born) × 100 External abnormalities (%) = (No. of pups with external abnormalities/No. of liveborn pups) × 100 Sex ratio = No. of liveborn male pups/(No. of liveborn male pups + No. of liveborn female pups) |
|
Viability index (%) = (No. of surviving pus on day 4 after birth/No. of liveborn pups on day 0 after birth) × 100
|
|
Endpoint
|
Generation
|
Sex
|
Effect level
|
Based on
|
Basis for effect level / Remarks
|
|
NOAEL
|
P
|
male/female
|
1000 mg/kg bw/day (actual dose received)
|
no effects on reproduction
|
|
|
LOAEL
|
P
|
male/female
|
100 mg/kg bw/day (actual dose received)
|
(See repeated dose toxicity)
|
|
|
NOAEL
|
F1
|
male/female
|
100 mg/kg bw/day (actual dose received)
|
Low body weight of pups at 300 mg/kg bw/day
|
|
yes (see 7.5.1 Repeated dose toxicity: oral.001)
|
|
yes (see 7.5.1 Repeated dose toxicity: oral.001)
|
|
no effects
|
|
not examined
|
|
no effects (on reproductive organs)
|
|
no effects (on reproductive organs)
|
|
no effects (on reproductive organs)
|
|
no effects (on reproductive organs)
|
|
no effects
|
|
no effects
|
|
yes (Low values of body weights of male and female pups were observed on postnatal day (PND) 4 at 300 mg/kg bw/day, and on PND 0 and PND 4 at 1000 mg/kg bw/day.)
|
|
not examined
|
|
not examined
|
|
no effects
|
|
not examined
|
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF31127 -54 -5d.pdf |
|
The NOAELs for rat reproductive toxicity and developmental toxicity were determined to be 1000 mg/kg bw/day and 100 mg/kg bw/day, respectively.
|
In the combined repeated oral dose toxicity study (0, 100, 300, and 1,000 mg/kg bw/day) with the reproduction/developmental toxicity screening test (OECD TG 422), no effects were found on reproductive parameters up to 1,000 mg/kg bw/day. The body weights of male and female pups decreased on postnatal day (PND) 4 at 300 mg/kg bw/day and higher, with decreased body weights observed for both sexes on PND 0 at 1,000 mg/kg bw/day. The no observed adverse effect levels (NOAELs) for rat reproductive toxicity and developmental toxicity were determined to be 1,000 mg/kg bw/day and 100 mg/kg bw/day, respectively. |
|
UUID
|
IUC4-b036ff75-0f3c-323b-b200-ed5f46cf5101
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
XML Transformation V4.0 Plug-In
|
|
|
Date
|
2011-06-23 11:55:01 JST
|
|
|
Remarks
|
Successfully migrated to IUCLID 5.5 format.
|
|
Legal entity name
|
National Institute of Health Sciences
|
|
Flags
|
IT system
|
ID
|
Remarks
|
|
LEO
|
10767
|
||
|
IUCLID4
|
16558402024DIV750
|
|
Address
|
1-18-1 kamiyoga
|
|
Address
|
Setagaya-ku
|
|
Postal code
|
158-8501
|
|
Town
|
Tokyo
|
|
Country
|
Japan
|
|
Organisation
|
National Institute of Health Sciences
|
|
Department
|
Division of Risk Assessment
|
|
Title
|
Dr.
|
|
First name
|
Akihiko
|
|
Last name
|
Hirose
|
|
Address
|
1-18-1 Kamiyoga
|
|
Address
|
Setagaya-ku
|
|
Postal code
|
158-8501
|
|
Town
|
Tokyo
|
|
Country
|
Japan
|
|
UUID
|
IUC5-9a63fecd-2bcb-4b40-abf4-b43e1c6090c7
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-10-30 11:33:45 JST
|
|
|
Remarks
|
|
Reference substance name
|
2,3,4,4'-Tetrahydroxybenzophenone
|
|
CAS number
|
31127-54-5
|
|
Molecular formula
|
31127-54-5
|
|
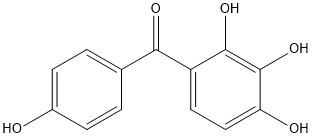
Structural formula
|
|