

|
|
Printing Date
|
2017-02-15 16:16:35 JST
|
|
Restriction of specific regulatory purposes
|
|
|
Confidentiality
|
|
|
Name
|
2-Nitro-p-cresol
|
|
Legal entity owner
|
National Institute of Health Sciences / Tokyo / Japan
|
|
UUID
|
IUC5-b0a94a30-717c-4d02-af0c-412c12b4d472
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2016-12-21 15:08:10 JST
|
|
|
Remarks
|
|
Chemical name
|
2-Nitro-p-cresol
|
|
Legal entity
|
|
|||||||||||||
|
UUID
|
IUC5-4b770d28-4eb4-4888-b89e-a27937fe34e8
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 15:32:16 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
The study was conducted in accordance with Test Guidelines and under GLP
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
publication
|
MHLW
|
2011
|
Twenty-eight-day Repeat Dose Oral Toxicity Test of 2-Nitro-p-cresol in Rats
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
Research institute for animal science in biochemistry and toxicology (RIAS)
|
|
data published
|
|
subacute
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
other guideline: other guideline: Guideline for 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan)
|
|
|
equivalent or similar to
|
OECD Guideline 407 (Repeated Dose 28-Day Oral Toxicity in Rodents)
|
|
yes
|
|
yes
|
|
- Name of test material (as cited in study report): 2-Nitro-p-cresol
- Analytical purity: 99.8% - Lot No.: FBR01 - Storage condition of test material: at a cold (temperature 2-6 ℃) and dark place, with airtight stopper. - Stability under test conditions: The stability of test material was identified by analysis of the remainder. |
|
rat
|
|
other: Crl:CD(SD)
|
|
male/female
|
|
TEST ANIMALS
- Source: Charles River Japan, Inc. - Age at study initiation: 5 weeks old - Weight at study initiation: male 161 g (146-173 g), female 144 g (130-154 g) - Housing: Animals were individually housed in a metallic cage with wire mesh bottoms - Diet: Solid feed (MR stock: Nosan Corporation) was given ad libitum. - Water: Tap water was given ad libitum. - Acclimation and quarantine period:7-8 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 22±3 (actual temperature: 22.0-22.6 ℃) - Humidity (%): 55±10% (actual humidity: 55-62%) - Air changes (per hr): 10-15 - Photoperiod (hrs dark / hrs light): 12 hr dark/12 hr light (light: 7:00~19:00) |
|
oral: gavage
|
|
olive oil
|
|
yes
|
|
28 days
|
|
once a day
|
|
0, 15, 60, 250, 1000 mg/kg bw/day
|
|
|
Basis
|
actual ingested
|
|
10/sex (0, 1000 mg/kg bw/day)
5/sex (15, 60, 250 mg/kg bw/day) |
|
yes, concurrent vehicle
|
|
- Dose selection rationale: Doses in this test were set based on the results of the following study: 14-day repeated dose oral toxicity test (Crl:CD(SD) rats, doses: 0 (olive oil), 10, 30, 100, 250, 500 or 1000 mg/kg bw/day). At 500 mg/kg/day and higher, sedation and salivation, and tendency of urine oxidation were observed in both sexes. At 1000 mg/kg/day, anemia and changes in liver functions were observed. At 250 mg/kg/day and higher, an increasing tendency on the liver weight was observed in both sexes. On the basis of these effects, a dose level of 1000 mg/kg was selected as the maximum dose expecting to induce the toxic changes, and then dose levels of 250, 60 and 15 mg/kg bw/day were selected, in accordance with a common ratio of approximately 4.
- Rationale for animal assignment (if not random): Body weight-balanced randomization - Post-exposure recovery period in satellite groups: 14 days |
|
CLINICAL OBSERVATIONS: Yes
- Time schedule: every day during the administration (4 times a day) and recovery periods (at least once a day) DETAILED CLINICAL OBSERVATIONS: Yes The functional observational battery testing (FOB) was performed on all animals. Among the measures in the FOB, detailed clinical observations were made before the initiation of dosing. Thereafter, detailed clinical observations were made once a week in dosing and recovery periods. Sensory motor reflexes, forelimb and hindlimb grip strengths, and motor activity were measured on week 4 of administration period (main/recovery group animals) and week 2 of recovery period (recovery group animals). BODY WEIGHT: Yes - Time schedule for examinations: Before administration (on days 1, 7, 14, 21 and 28 of the administration period, days 7 and 14 of the recovery period) and the necropsy days after completion of every period. FOOD CONSUMPTION AND COMPOUND INTAKE (if feeding study): - Food consumption: Yes. Once a week for 24-h (males: on days 5, 12, 19 and 26 of the administration period and days 5 and 12 of the recovery period. females: on days 4, 11, 18 and 25 of the administration period and days 4 and 11 of the recovery period) OPHTHALMOSCOPIC EXAMINATION: No HAEMATOLOGY: Yes - Time schedule for collection of blood: the after completion of the administration and recovery periods - Anaesthetic used for blood collection: ether - Animals fasted: Yes (overnight) - How many animals: all animals CLINICAL CHEMISTRY: Yes - Time schedule for collection of blood: the day after completion of the administration and recovery periods - Anaesthetic used for blood collection: ether - Animals fasted: Yes (overnight) - How many animals: all animals URINALYSIS: Yes - Time schedule for collection of urine: on weeks 4 of the administration period and weeks 2 of the recovery period. - Metabolism cages used for collection of urine: Yes NEUROBEHAVIOURAL EXAMINATION: No |
|
GROSS PATHOLOGY: Yes
ORGAN WEIBHT: Yes [brain, pituitary gland, thyroid, adrenal, spleen, heart, liver, kidney, thymus, testis, epididymis, ovary] HISTOPATHOLOGY: Yes [brain (cerebrum, cerebellum and medulla oblongata), pituitary gland, spinal cord (cervical, thoracical, lumber), thymus, thyroid (including parathyroid), adrenal glands, spleen, heart, stomach, liver, duodenum, jejunum, ileum (including Peyer's patches), cecum, colon, rectal, mesenteric lymph nodes, submandibular lymph nodes, trachea, lung, kidney, bladder, testis, epididymis, prostate, seminal vesicles, ovary, uterus, vagina, eye, bone marrow (femur) and the sciatic nerve. (see tables in the study report.) |
|
As for parametric data (grip strength, locomotor activity, body weight, body weight gain, food consumption, hematology and clinical chemistry data, organ weights), the values of means and standard deviations were calculated per group. When more than three groups exist in the test group, Bartlett test for variance was done, and if the variance was homogenous, ANOVA was applied. If the variance was not homogenous or data was non-parametric (differential WBC percentage, urinalysis data), Kruskal-Wallis rank sum test was used. Consequently, if the result was significant, Dunnett multiple comparison or Dunnett-typed method was used for detection of statistical significance against control group. When the number of the test group was two, F-test was used as for parametric data. Then, student's t-test or Aspin-Welch's t-test was applied depending on the result of homogeneity of variance. While, as for non-parametric data, Man-Whitney's U-test was applied. Furthermore, as for categorized data (incidence of abnormal findings in clinical observation, detailed observation, sensory functional examination, necropsy and histopathology), Fischer's exact test was used. In any tests, level of significance was set at 5%.
|
|
Endpoint
|
Effect level
|
Based on
|
Sex
|
Basis for effect level / Remarks
|
|
NOAEL
|
60 mg/kg bw/day (actual dose received)
|
test mat.
|
male/female
|
At 250 mg/kg bw/day and higher, sedation and ptosis were observed in both sexes. Increase in the liver weight was observed at 250 mg/kg bw/day and higher in females and at 1,000 mg/kg bw/day in males. Histopathological examinations revealed hypertrophy of hepatocytes at 250 mg/kg bw/day and higher in females.
|
|
yes (see Details on results)
|
|
no effects
|
|
no effects
|
|
yes (see Details on results)
|
|
yes (see Details on results)
|
|
yes (see Details on results)
|
|
no effects (see Details on results)
|
|
yes (see Details on results)
|
|
yes (see Details on results)
|
|
yes (see Details on results)
|
|
CLINICAL SIGNS AND MORTALITY
At 250 mg/kg bw/day and higher, sedation and ptosis were observed in both sexes. Transient salivation was observed in both sexes at 1,000 mg/kg bw/day. Soiled fur in one female and reddish tear in one male were observed at 1,000 mg/kg bw/day. NEUROBEHAVIOUR Clinical signs in detailed observation: No effects. Sensory/reflex function test: No effects. Grip strength: In the recovery period, high value of hindlimb strength in males and low value of forelimb strength in female were observed. (These were within background date.) Motor activity: No effects. BODY WEIGHT AND WEIGHT GAIN: No effects. FOOD CONSUMPTION: No toxicological effects. HAEMATOLOGY At 1,000 mg/kg bw/day, low values of Hb, Ht and MCHC, and high values of Ret were observed in males and females, and high value of APTT was observed in females. At the end of recovery period, high values of MCV and MCH, and low value of MCHC were observed in males. CLINICAL CHEMISTRY At 1,000 mg/kg bw/day, high values of Alb, A/G, T-Cho, and K in males, and high values of γ-GTP and T-Bil in females were observed. At the end of recovery period, high value of Na was observed in males. URINALYSIS Pale yellow color was observed at 250 mg/kg bw/day and higher in males and females. At 1,000 mg/kg bw/day, low value of pH were observed in males and females. In the recovery period, low value of specific gravity was observed in males. ORGAN WEIGHTS At 250 mg/kg bw/day and higher, sedation and ptosis were observed in both sexes. Increase in the liver weight was observed at 250 mg/kg bw/day and higher in females and at 1,000 mg/kg bw/day in males. Furthermore, increases in the kidney weight in males and spleen weight in both sexes were observed at 1,000 mg/kg bw/day. GROSS PATHOLOGY At 1,000 mg/kg bw/day, blackish color of the spleen was observed in males and females at the ends of administration and recovery periods. HISTOPATHOLOGY: NON-NEOPLASTIC Histopathological examinations revealed hyperostosis metaphysis of the femur in females at 200 mg/kg bw/day. Furthermore, at 1,000 mg/kg bw/day, hyperkeratosis of the mucosal epithelium in the forestomach, grade enhancement of regeneration of the tubular epithelium, dilatation of the renal tubules in the cortex, and cyst-like extension of the collecting duct in the kidney, and hyperostosis metaphysis of the femur were observed in both sexes. Additionally in males at 1,000 mg/kg bw/day, degeneration of nerve fibers in the sciatic nerve and atrophy of muscle fibers in the skeletal muscle were observed. And in females at 1,000 mg/kg bw/day, squamous cell hyperplasia of the boundary edge in the stomach was observed. These changes tended to resolve after the recovery period. (See tables in the full report for more details) |
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF119 -33 -5b.pdf |
A 28-day repeated-dose toxicity test was performed according to the Japanese guideline (similar to OECD TG 407). Male and female rats (5 animals/sex/dose) were administered 2-nitro-p-cresol at 0, 15, 60, 250, and 1,000 mg/kg bw/day. In addition, both sexes (5 animals/sex/dose) were administered 0 and 1,000 mg/kg bw/day of this substance for 28 days and examined after a 14-day recovery period. At 250 mg/kg bw/day and higher, sedation and ptosis were observed in both sexes. Increase in the liver weight was observed at 250 mg/kg bw/day and higher in females andat 1,000 mg/kg bw/day in males. Furthermore, increases in the kidney weight in males and spleen weight in both sexes were observed at 1,000 mg/kg bw/day. Histopathological examinations revealed hypertrophy of hepatocytes at 250 mg/kg bw/day and higher in females. At 1,000 mg/kg bw/day, increase in the extramedullary hematopoiesis and brown pigmentation in the spleen was observed in both sexes. Additionally in males, hypertrophy of hepatocytes in the liver was observed at 1,000 mg/kg bw/day. Moreover, an increase in hyaline droplets containing α2u-globulin in the renal proximal tubular epithelium in the kidney was observed in males at the same dose.These changes, exceptbrown pigmentation in the spleen,tended to resolve after the recovery period.On the basis of these effects, NOAEL for repeated-dose toxicity was determined to be 60 mg/kg bw/day in male and female rats. |
|
7.5.1 Repeated dose toxicity: oral.002
|
|
UUID
|
IUC5-c2b1b0b7-0568-4483-b21c-54cfa1d1f5a7
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 15:35:18 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
GLP guideline study
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW Japan
|
2012
|
A reproduction/developmental toxicity screening test in rats treated orally with 2-nitro-p-cresol
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
BoZo Research Center
|
|
data published
|
|
7.8.1 Toxicity to reproduction.001
|
|
combined repeated dose and reproduction / developmental screening
|
|
no
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
other guideline: OECD TG 421: Reproduction/developmental toxicity screening test
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
119-33-5
|
|
- Name of test material (as cited in study report): 2-nitro-p-cresol
- Purity: 99.6% - Lot/batch No.: FHD01 - Stability under test conditions: Stable - Storage condition of test material: a cool (3-6 °C) and dark place (in a refrigerator), with an airtight stopper - Dosing solution storage condition: under a cool (3-6 °C) place (in a refrigerator), in a brown glass bottle - Other: The dosing solution was used within 7 days of preparation. |
|
rat
|
|
other: Crl: CD(SD)
|
|
male/female
|
|
TEST ANIMALS
- Source: Charles River Laboratories Japan, Inc. Tsukuba - Age at study initiation: 10 weeks - Weight at study initiation: Males: 392-474 (average 427) g; Females: 238-297 (average 270) g - Housing: Steel wire-mesh cage (250 mm x 350 mm x 200 mm ) - Diet: ad libitum - Water: ad libitum - Acclimation period: 19 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 20-24 - Humidity (%): 33-69 - Air changes: 10-15 times / hr - Photoperiod: 12 hrs dark / 12 hrs light (07:00-19:00) |
|
oral: gavage
|
|
olive oil
|
|
yes
|
|
(P) Males: 42 days including 14 days pre-mating and mating periods, and thereafter 14 days (P) Females: 42-47 days including 14 days pre-mating, mating and gestation periods, and the days until day 4 of lactation. Infertile females: 40-53 days
|
|
Once/day, 7days/week
|
|
0 (vehicle), 60, 250, and 1000 mg/kg bw/day
|
|
|
Basis
|
actual ingested
|
|
12 animals/sex/dose
|
|
yes, concurrent vehicle
|
|
- Dose selection rationale: Doses in this test were set based on the results of the following study: 28-day repeated dose oral toxicity test (doses: 0, 15, 60, 250, and 1000 mg/kg bw/day). At 250 mg/kg bw/day and higher, sedation and ptosis were observed in both sexes. Increase in the liver weight was observed at 250 mg/kg bw/day and higher in females and at 1,000 mg/kg bw/day in males. Furthermore, increases in the kidney weight in males and spleen weight in both sexes were observed at 1,000 mg/kg bw/day. Histopathological examinations revealed hypertrophy of hepatocytes at 250 mg/kg bw/day and higher in females. At 1,000 mg/kg bw/day, increase in the extramedullary hematopoiesis and brown pigmentation in the spleen was observed in both sexes. Additionally in males, hypertrophy of hepatocytes in the liver was observed at 1,000 mg/kg bw/day. Moreover, an increase in hyaline droplets containing α2u-globulin in the renal proximal tubular epithelium in the kidney was observed in males at the same dose. These changes, except brown pigmentation in the spleen, tended to resolve after the recovery period.
On the basis of these effects, a dose level of 1000 mg/kg was selected as the maximum dose expecting to induce the toxic changes, and then dose levels of 250 and 60 mg/kg bw/day were selected as a middle dose and a minimum dose levels, respectively, in accordance with a common ratio of approximately 4. - Rationale for animal assignment (if not random): Body weight-balanced randomization |
|
CAGE SIDE OBSERVATIONS: Yes
- Time schedule: Males and females: 3 times/day BODY WEIGHT: Yes - Time schedule for examinations: Males: Days 1, 4, 8, 11, 15, 22, 25, 29, 32, 36, 39, 42, and the day of necropsy Females: Twice a week during the precopulation period (days 1, 4, 8, 11, and 15); gestation days 0, 4, 7, 11, 14, 17, and 20; lactation days 0 and 4; and the day of necropsy. For unmating females, 18, 22 and 25 in the mating period FOOD CONSUMPTION: Yes Males: Days 1, 4, 8, 11, 15, 32, 36, 39, and 42 in dosing period Females: Days 1, 4, 8, 11, and 15; gestation days 1, 4, 7, 11, 14, 17, and 20; lactation days 2 and 4 HAEMATOLOGY: No CLINICAL CHEMISTRY: No URINALYSIS: No |
|
GROSS PATHOLOGY: Yes (see tables)
HISTOPATHOLOGY: Yes (epididymis, prostate, seminal vesicle, testis, ovary, uterus, vagina, and gross abnormal sites) |
|
Organ weight: Testes and epididymides
|
|
3 or more groups: The data were analyzed for homogeneity of variance by the Bartlett test. If variances were homogeneous, data was analyzed by the Dunnett test, whereas heterogeneous data was analyzed by the steel test (p<0.05, two-sided).
2 groups: The data were analyzed for homogeneity of variance by the F test. If variances were homogeneous, data was analyzed by the Student t test, whereas heterogeneous data was analyzed by the Aspin-Welch t test (p<0.05, two-sided). |
|
Endpoint
|
Effect level
|
Based on
|
Sex
|
Basis for effect level / Remarks
|
|
NOAEL
|
250 mg/kg bw/day (actual dose received)
|
test mat.
|
male/female
|
At 1,000 mg/kg bw/day, ptosis and decreased locomotor activity were observed in both sexes. At the same dose, histopathological examinations revealed centrilobular hypertrophy of hepatocytes in the liver and increased extramedullary hematopoiesis in the spleen in both sexes.
|
|
yes (At 1,000 mg/kg bw/day, ptosis and decreased locomotor activity were observed in both sexes.)
|
|
no effects
|
|
no effects
|
|
not examined
|
|
not examined
|
|
not examined
|
|
not examined
|
|
not examined
|
|
not examined
|
|
not examined
|
|
no effects
|
|
yes (see tables in the full report.)
|
|
yes (see tables in the full report.)
|
|
not examined
|
|
At 1,000 mg/kg bw/day, histopathological examinations revealed centrilobular hypertrophy of hepatocytes in the liver and increased extramedullary hematopoiesis in the spleen in both sexes.
|
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF119 -33 -5c.pdf |
|
In this study, NOAEL for repeated-dose toxicity was determined to be 250 mg/kg bw/day in male and female rats.
|
A reproduction/developmental toxicity screening test was performed according to OECD TG 421. Male and female rats (12 animals/sex/dose) were administered 2-nitro-p-cresol at 0, 60, 250, and 1,000 mg/kg bw/day. Males were dosed for 42 days, including a 14 day pre-mating and mating periods. Females were dosed for 42–47 days, including a 14 day pre-mating, mating, and gestation periods, and the time until lactation day 4. At 1,000 mg/kg bw/day, ptosis and decreased locomotor activity were observed in both sexes. At the same dose, histopathological examinations revealed centrilobular hypertrophy of hepatocytes in the liver and increased extramedullary hematopoiesis in the spleen in both sexes. On the basis of these changes, NOAEL for repeated-dose toxicity was determined to be 250 mg/kg bw/day in male and female rats. |
|
7.5.1 Repeated dose toxicity: oral.001
|
|
UUID
|
IUC5-052223da-12ec-41a9-9c66-24acf10465ce
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 15:38:25 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
GLP guideline study
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW Japan
|
2007
|
Reverse mutation test of 2-nitro-p-cresol in Bacteria
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
Bozo Research Center Inc.
|
|
data published
|
|
gene mutation
|
|
bacterial reverse mutation assay (e.g. Ames test)
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 471 (Bacterial Reverse Mutation Assay)
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
119-33-5
|
|
crystalline
|
|
- Name of test material (as cited in study report): 2-Nitro-p-cresol
- Analytical purity: 99.8% - Lot/batch No.:FBR01 - Stability under test conditions: Stable - Storage condition of test material: A cool and dark place, with an airtight stopper |
|
Species/strain
|
S. typhimurium TA 1535, TA 1537, TA 98, TA 100 and E. coli WP2
|
|
Metabolic activation
|
with and without
|
|
Metabolic activation system
|
S9 mix
|
|
Dose–range finding test (–S9 mix and +S9 mix): 0 (vehicle), 1.22, 4.88, 19.5, 78.1, 313, 1250, and 5000 μg/plate;
Main bacterial reverse mutation test (–S9 mix and +S9 mix): 0 (vehicle), 39.1-5000 μg/plate [1st]. 0 (vehicle), 19.5-5000 μg/plate [2nd]. 0 (vehicle), 19.5-1250 μg/plate [3rd, TA98]. |
|
- Vehicle(s)/solvent(s) used: DMSO
- Justification for choice of solvent/vehicle: The test substance was soluble in DMSO, but not in water. |
|
Negative controls
|
no
|
||||
|
Solvent / vehicle controls
|
yes
|
||||
|
True negative controls
|
yes (tests without all strains)
|
||||
|
Positive controls
|
yes
|
||||
|
Positive control substance
|
|
|
METHOD OF APPLICATION: Preincubation
DURATION - Preincubation period: 20 min - Exposure duration: ca. 50 hours NUMBER OF REPLICATIONS: 3 DETERMINATION OF CYTOTOXICITY - Method: Cell growth |
|
Criteria for determining a positive result were as follows; A 2–fold or more increase in the number of revertant colonies compared with the solvent control, a concentration–related increase in the number of revertant colonies, and a reproducible increase in the number of revertant colonies.
|
|
No statistic method was used for judging of results.
|
|
Species/strain
|
S. typhimurium TA 1535, TA 1537, TA 98 and TA 100
|
|
Metabolic activation
|
with and without
|
|
Test system
|
all strains/cell types tested
|
|
Genotoxicity
|
negative
|
|
Cytotoxicity
|
yes (see tables.)
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
Species/strain
|
E. coli WP2 uvr A
|
|
Metabolic activation
|
with and without
|
|
Test system
|
all strains/cell types tested
|
|
Genotoxicity
|
negative
|
|
Cytotoxicity
|
yes (see tables.)
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
TEST-SPECIFIC CONFOUNDING FACTORS
- Precipitation: Precipitation was not observed on any plates with/without metabolic activation. - Other effects: coloring was observed on plates with concentration of 1250 μg/plate or more with/without metabolic activation in range-finding studies. RANGE-FINDING/SCREENING STUDIES: In range-finding studies, growth inhibition was observed on plates with concentration of 1250 μg/plate or more in all S. typhimurium strains with/without metabolic activation and on plates with concentration of 5000 μg/plate in all E.coli strains with/without metabolic activation. COMPARISON WITH HISTORICAL CONTROL DATA: In all test conditions and in all tested strains, the number of revertant colonies of solvent controls and positive controls were within the range of historical control data. |
Figures and Tables are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF119 -33 -5e.pdf |
|
negative
|
In a bacterial reverse mutation assay usingS. typhimuriumTA100, TA1535, TA98, and TA1537 andE. coliWP2uvrA(OECD TG 471), 2-nitro-p-cresol was negative with or without metabolic activation. |
|
UUID
|
IUC5-05fb6ad5-c084-4f39-96a2-3ec8deffac01
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 15:41:24 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
OECD Test Guideline study under GLP condition
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW, Japan
|
2007
|
In Vitro Chromosomal Aberration Test of 2-nitro-p-cresol on Cultured Chinese Hamster Cells.
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
BoZo Research Center
|
|
data published
|
|
chromosome aberration
|
|
in vitro mammalian chromosome aberration test
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 473 (In vitro Mammalian Chromosome Aberration Test)
|
no
|
|
according to
|
JAPAN: Guidelines for Screening Mutagenicity Testing Of Chemicals
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
119-33-5
|
|
- Name of test material (as cited in study report): 2-Nitro-p-cresol
- Analytical purity: 99.8% - Lot/batch No.:FBR01 - Stability under test conditions: Stable - Storage condition of test material: A cool and dark place, with an airtight stopper |
|
Chromosome
|
|
Species/strain
|
other: Chinese hamster lung(CHL/IU) cells
|
|
Metabolic activation
|
with and without
|
|
Metabolic activation system
|
rat liver, induced by phenobarbital and 5,6-benzoflavone
|
|
-S9 mix (short-term treatment): 0, 25.0, 50.0, 100, 200, 400 ug/mL
+S9 mix (short-term treatment): 0, 25.0, 50.0, 100, 200, 400 ug/mL +S9 mix (short-term treatment, confirmation test): 0, 300, 400, 500, 600, 700, 800 ug/mL -S9 mix (continuous treatment, 24 h): 0, 25.0, 50.0, 100, 200, 400 ug/mL -S9 mix (continuous treatment, 48 h): 0, 25.0, 50.0, 100, 200, 400 ug/mL |
|
- Vehicle(s)/solvent(s) used: DMSO
|
|
Negative controls
|
no
|
||
|
Solvent / vehicle controls
|
yes
|
||
|
True negative controls
|
no
|
||
|
Positive controls
|
yes
|
||
|
Positive control substance
|
|
||
|
Remarks
|
mitomycin C (without S9 mix), cyclophosphamide (with S9 mix)
|
|
METHOD OF APPLICATION: Exposure duration: [continuous treatment]: 24, 48 hrs [short-term treatment]:6 hrs + 18 hr
SPINDLE INHIBITOR: Colcemid NUMBER OF REPLICATIONS: 2 NUMBER OF CELLS EVALUATED: 200 cells / dose DETERMINATION OF CYTOTOXICITY - Method: relative total growth |
|
For the evaluation of the frequencies of structural aberrations and of polyploidy induced, the following criteria were employed.
Appearance incidence of cells with chromosomal aberrations: Negative (-): < 5%; equivocal (±): 5-10%; positive (+): > 10%. Finally, the substance is positive when the incidence is considered to be dose-related and reproducible. |
|
not used.
|
|
Species/strain
|
other: Chinese hamster lung (CHL/IU) cells
|
|
Metabolic activation
|
with
|
|
Genotoxicity
|
positive (structural aberration)
|
|
Cytotoxicity
|
yes 50% cell growth inhibition: 190.0 ug/mL (short)
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
|
Species/strain
|
other: Chinese hamster lung (CHL/IU) cells
|
|
Metabolic activation
|
without
|
|
Genotoxicity
|
negative
|
|
Cytotoxicity
|
yes 50% cell growth inhibition: 192.3 ug/mL (short), 252.6 ug/mL (24h continuous) and 200.0 ug/mL (48h continuous)
|
|
Vehicle controls valid
|
yes
|
|
Negative controls valid
|
not examined
|
|
Positive controls valid
|
yes
|
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF119 -33 -5f.pdf |
Anin vitrochromosomal aberration test using CHL/IU cells (OECD TG 473) showed positive result with metabolic activation. |
|
UUID
|
IUC5-40365d06-5dac-40cf-a914-51fc7eacba50
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 15:42:10 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
|
Reliability
|
1 (reliable without restriction)
|
|
Rationale for reliability incl. deficiencies
|
OECD Guideline study under GLP condition
|
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW, Japan
|
2011
|
Micronucleous test of 2-nitro-p-cresol on mouse
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
Bozo Research Center
|
|
chromosome aberration
|
|
micronucleus assay
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
OECD Guideline 474 (Mammalian Erythrocyte Micronucleus Test)
|
no data
|
|
according to
|
other guideline: Testing Methods for New Chemical Substances etc.
|
no data
|
|
yes (incl. certificate)
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
41267-43-0
|
|
- Name of test material (as cited in study report): 2-Nitro-p-cresol
- Analytical purity: 99.6% - Lot/batch No.:FHD01 - Stability under test conditions: Stable - Storage condition of test material: A cool and dark place (in a refrigerator, 3-6 ℃), with an airtight stopper |
|
mouse
|
|
other: Crlj: CD1(ICR)
|
|
male
|
|
TEST ANIMALS
- Source: Charles River Laboratories Japan, Inc. Atsugi Farm Center - Age at study initiation: 8 weeks old - Weight at study initiation: 31.2-36.1 g - Assigned to test groups randomly: yes - Housing: White flake (Charles River Japan, Inc.) in plastic cage (W 155 x K 245 x H 150mm: Clea Japan, Inc.) - Diet: ad libitum - Water: ad libitum - Acclimation period: 8 days ENVIRONMENTAL CONDITIONS - Temperature (°C):21-23 - Humidity (%):47-67 - Air changes: 10-15/h - Photoperiod: 12 h dark/ 12 h light (light time: 7:00 to 19:00) |
|
oral: gavage
|
|
- Vehicle(s)/solvent(s) used: olive oil
- Concentration of test material in vehicle: 0, 25, 50, and 100 mg/mL - Amount of vehicle (if gavage or dermal): 10 mL/kg bw - Lot/batch no. (if required): 0420, 0929 |
|
PREPARATION OF DOSING SOLUTIONS:
Dosing solutions were prepared by dissolving the test substance in olive oil. They were used within 6 days. |
|
24 h
|
|
Twice, 24 h interval
|
|
0 (vehicle), 250, 500, and 1000 mg/kg bw
|
|
|
Basis
|
actual ingested
|
|
5 males/dose
|
|
yes, concurrent vehicle
|
|
Mitomycin C (MMC)
- Justification for choice of positive control: MMC is widely used in the micronucleus test and is one of the positive control materials exemplified and recommended in the applicable guidelines. - Route of administration: intraperitoneal injection - Doses / concentration: 1 mg/kg bw |
|
Polychromatic erythrocytes from the femur bone marrow
|
|
TREATMENT AND SAMPLING TIMES (in addition to information in specific fields): Cells for specimen were collected 24 h after the administration.
DETAILS OF SLIDE PREPARATION: Cell suspensions were expanded on the slide glass and dried. The expanded cells were stained using a cover glass with a small amount of acridine orange solution (40ug/mL). METHOD OF ANALYSIS: fluorescence microscopy, blind method |
|
Criterion for determining a positive result: A dose-related increase in the number of micronucleated cells.
|
|
The number of micronucleated polychromatic erythrocytes was determined by the Kastenbaum and Bowman method, and Cochran Armitage test;
Ratio of polychromatic erythrocytes to whole erythrocytes by Bartlett's test and Dunnett's test |
|
Sex
|
male
|
|
Genotoxicity
|
negative
|
|
Vehicle controls valid
|
yes
|
|
Positive controls valid
|
yes
|
|
RESULTS OF RANGE-FINDING STUDY
- Dose range: 250, 500, 1000, 2000 mg/kg bw for males and females - Clinical signs of toxicity in test animals: Death was observed in one male and one female at 2000 mg/kg bw. Colored urine and lowered body weight were observed in all animals dosed. - Harvest times: 24 h after the treatment RESULTS OF DEFINITIVE STUDY - Induction of micronuclei (for Micronucleus assay): Males: The number of micronucleated cells in all dosed groups was within the range of control. Females: The study was not conducted because no sex differences were found in the preliminary study. - Ratio of PCE/NCE (for Micronucleus assay): Ratios for dose levels, 0, 250, 500, and 1000 mg/kg bw/day: 0.13%, 0.13%, 0.16%, and 0.11%; Positive control: 2.54% |
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF119 -33 -5g.pdf |
|
negative
|
|
The test substance did not produce micronuclei in the immature erythrocytes of the test species.
|
the result of anin vivomicronucleus study (OECD TG 474) were negative up to the maximum tolerated dose (1,000 mg/kg bw/day for 2 days) in mice. |
|
UUID
|
IUC5-8f5a4886-495c-4d75-a80e-240dc9bce661
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2017-02-15 15:43:40 JST
|
|
|
Remarks
|
|
Purpose flag
|
key study
|
||
|
Study result type
|
experimental result
|
||
|
Reliability
|
1 (reliable without restriction)
|
||
|
Rationale for reliability incl. deficiencies
|
OECD Test Guideline study under GLP condition
|
||
|
Reference type
|
Author
|
Year
|
Title
|
Bibliographic source
|
Testing laboratory
|
Report no.
|
Owner company
|
Company study no.
|
Report date
|
|
study report
|
MHLW, Japan
|
2012
|
A reproduction/developmental toxicity screening test in rats treated orally with 2-nitro-p-cresol
|
available in the web of Japan Existing Chemical Data Base (JECDB) at http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp
|
BoZo Research Center
|
|
data published
|
|
7.5.1 Repeated dose toxicity: oral.002
|
|
screening
|
|
no
|
|
Qualifier
|
Guideline
|
Deviations
|
|
according to
|
other guideline: OECD TG 421: Reproduction/developmental toxicity screening test
|
no
|
|
yes
|
|
yes
|
|
Identifier
|
Identity
|
|
CAS number
|
119-33-5
|
|
- Name of test material (as cited in study report): 2-nitro-p-cresol
- Purity: 99.6% - Lot/batch No.: FHD01 - Stability under test conditions: Stable - Storage condition of test material: a cool (3-6 °C) and dark place (in a refrigerator), with an airtight stopper - Dosing solution storage condition: under a cool (3-6 °C) place (in a refrigerator), in a brown glass bottle - Other: The dosing solution was used within 7 days of preparation. |
|
rat
|
|
other: Crl:CD(SD)
|
|
male/female
|
|
TEST ANIMALS
- Source: Charles River Laboratories Japan, Inc. Tsukuba - Age at study initiation: 10 weeks - Weight at study initiation: Males: 392-474 (average 427) g; Females: 238-297 (average 270) g - Housing: Steel wire-mesh cage (250 mm x 350 mm x 200 mm ) - Diet: ad libitum - Water: ad libitum - Acclimation period: 19 days ENVIRONMENTAL CONDITIONS - Temperature (°C): 20-24 - Humidity (%): 33-69 - Air changes: 10-15 times / hr - Photoperiod: 12 hrs dark / 12 hrs light (07:00-19:00) |
|
oral: gavage
|
|
olive oil
|
|
- M/F ratio per cage:1:1
- Length of cohabitation:up to 14 days - Proof of pregnancy: [vaginal plug / sperm in vaginal smear] referred to as [day 0] of pregnancy |
|
yes
|
|
(P) Males: 42 days including 14 days pre-mating and mating periods, and thereafter 14 days (P) Females: 42-47 days including 14 days pre-mating, mating and gestation periods, and the days until day 4 of lactation. Infertile females: 40-53 days
|
|
Once/day, 7days/week
|
|
0 (vehicle), 60, 250, and 1000 mg/kg bw/day
|
|
|
Basis
|
actual ingested
|
|
12 animals/sex/dose
|
|
yes, concurrent vehicle
|
|
CAGE SIDE OBSERVATIONS: Yes
- Time schedule: Males and females: 3 times/day BODY WEIGHT: Yes - Time schedule for examinations: Males: Days 1, 4, 8, 11, 15, 22, 25, 29, 32, 36, 39, 42, and the day of necropsy Females: Twice a week during the precopulation period (days 1, 4, 8, 11, and 15); gestation days 0, 4, 7, 11, 14, 17, and 20; lactation days 0 and 4; and the day of necropsy. For unmating females, 18, 22 and 25 in the mating period FOOD CONSUMPTION: Yes Males: Days 1, 4, 8, 11, 15, 32, 36, 39, and 42 in dosing period Females: Days 1, 4, 8, 11, and 15; gestation days 1, 4, 7, 11, 14, 17, and 20; lactation days 2 and 4 OTHER: Females: Numbers of corpus luteum and implantation site on the day of necropsy |
|
Vaginal smears were collected from all females in the main groups and microscopically examined every day from the day after the start of administration until the day copulation was confirmed. During the pre-mating administration period, vaginal smear pictures were classified as proestrus, estrus, metestrus or diestrus and examined for the frequency of estrus and interval between estruses (estrous cycle). During the mating period, vaginal smears were examined for the presence of sperm.
|
|
Parameters examined in P male parental generations: testes weight, epididymides weight
|
|
PARAMETERS EXAMINED:The following parameters were examined in F1 offspring [number and sex of pups, stillbirths, live births, postnatal mortality, presence of gross anomalies, and weight].
GROSS EXAMINATION OF DEAD PUPS: Yes, for external and internal abnormalities. |
|
SACRIFICE:
Male animals: Rats were euthanized by exsanguination under ether anesthesia on the day after the last administration. Maternal animals: Rats were euthanized by exsanguination under ether anesthesia on day 4 of lactation. GROSS PATHOLOGY: Yes (see tables) HISTOPATHOLOGY: Yes (epididymis, prostate, seminal vesicle, testis, ovary, uterus, vagina, and gross abnormal sites) ORGAN WEIGHTS, Yes: Testes and epididymis |
|
GROSS NECROPSY
- Gross necropsy consisted of external and internal examinations including the cervical, thoracic, and abdominal viscera. |
|
The data were analyzed for homogeneity of variance by the Bartlett test. If variances were homogeneous, data was analyzed by the Dunnett test, whereas heterogeneous data was analyzed by the Steel test (p<0.05, two-sided).
2 groups: The data were analyzed for homogeneity of variance by the F test. If variances were homogeneous, data was analyzed by the Student t test, whereas heterogeneous data was analyzed by the Aspin-Welch t test (p<0.05, two-sided). Especially, Implantation index, Stillborn index, Liveborn index, External abnormalities, Viability index: the Steel test (p<0.05 and <0.01, two-sided) Copulation index, Fertility index, Insemination index, Delivery index: Fisher's exact test (p<0.05 and <0.01, two-sided) |
|
Each parameter was determined by the following equations:
Copulation index (%) = (No. of copulated animals/No. of co-housed animals) × 100 Fertility index (%) = (No. of pregnant females/No. of copulated females) × 100 Insemination index (%) = (No. of pregnant females/No. of copulated males) × 100 Duration of gestation (days) = day 0 of lactation – day 0 of gestation Delivery index (%) = (No. of females delivered liveborn pups/No. of pregnant females) × 100 Implantation index (%) = (No. of implantation sites/No. of corpora lutea) × 100 Stillborn index (%) = (No. of stillborn pups/Total No. of pups born) × 100 Liveborn index (%) = (No. of liveborn pups/Total No. of pups born) × 100 External abnormalities (%) = (No. of pups with external abnormalities/No. of liveborn pups) × 100 Sex ratio = No. of liveborn male pups/(No. of liveborn male pups + No. of liveborn female pups) |
|
Viability index (%) = (No. of surviving pus on day 4 after birth/No. of liveborn pups on day 0 after birth) × 100
|
|
Endpoint
|
Generation
|
Sex
|
Effect level
|
Based on
|
Basis for effect level / Remarks
|
|
NOAEL
|
P
|
male/female
|
250 mg/kg bw/day (actual dose received)
|
||
|
NOAEL
|
F1
|
male/female
|
1000 mg/kg bw/day (actual dose received)
|
the highest dose tested
|
|
yes (see 7.5.1 Repeated dose toxicity: oral.002)
|
|
yes (see 7.5.1 Repeated dose toxicity: oral.002)
|
|
no effects
|
|
not examined
|
|
no effects (on reproductive organs)
|
|
no effects (on reproductive organs)
|
|
no effects (on reproductive organs)
|
|
no effects (on reproductive organs)
|
|
no effects
|
|
no effects
|
|
no effects
|
|
not examined
|
|
not examined
|
|
no effects
|
|
not examined
|
Figures and Tables (in English) are available in the following full report of the study. http://dra4.nihs.go.jp/mhlw_data/home/pdf/PDF119 -33 -5c.pdf |
|
NOAEL for the rat reproductive/developmental toxicity of 4-chlorobenzaldehyde was determined to be 200 mg/kg bw/day.
|
In the reproduction/developmental toxicity screening test (0, 60, 250, and 1,000 mg/kg bw/day) (OECD TG 421), no effects of this substance on reproductive and developmental parameters were observed at 1,000 mg/kg bw/day. NOAEL for the rat reproductive/developmental toxicity of 2-nitro-p-cresol was determined to be 1,000 mg/kg bw/day, the highest dose tested. |
|
UUID
|
ECB5-4c8b7908-3640-47d4-b274-fae78201d6fd
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
dra / National Institute of Health Sciences / Tokyo / Japan
|
|
|
Date
|
2016-12-21 15:07:54 JST
|
|
|
Remarks
|
|
Reference substance name
|
2-nitro-p-cresol
|
|
EC number
|
204-315-6
|
CAS number
|
119-33-5
|
|
EC name
|
2-nitro-p-cresol
|
||
|
Molecular formula
|
C7H7NO3
|
||
|
CAS number
|
119-33-5
|
|
4-methyl-2-nitrophenol
|
|
Name
|
2-nitro-p-cresol
|
|
Name
|
Phenol, 4-methyl-2-nitro-
|
|
OECD Category: m,p - Cresols
USEPA Category: Phenols |
|
Molecular formula
|
C7H7NO3
|
|
Molecular weight range
|
153.1354
|
|
SMILES notation
|
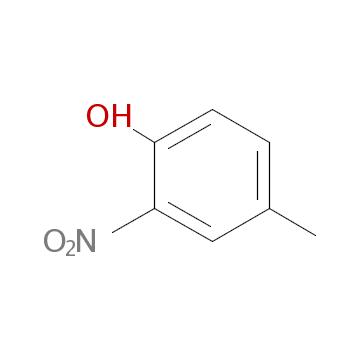
Cc1ccc(O)c(c1)[N+](=O)[O-]
|
|
InChI
|
InChI=1/C7H7NO3/c1-5-2-3-7(9)6(4-5)8(10)11/h2-4,9H,1H3
|
|
Structural formula
|
|
|
UUID
|
IUC4-b036ff75-0f3c-323b-b200-ed5f46cf5101
|
|
|
Dossier UUID
|
0
|
|
|
Author
|
XML Transformation V4.0 Plug-In
|
|
|
Date
|
2011-06-23 11:55:01 JST
|
|
|
Remarks
|
Successfully migrated to IUCLID 5.5 format.
|
|
Legal entity name
|
National Institute of Health Sciences
|
|
Flags
|
IT system
|
ID
|
Remarks
|
|
LEO
|
10767
|
||
|
IUCLID4
|
16558402024DIV750
|
|
Address
|
1-18-1 kamiyoga
|
|
Address
|
Setagaya-ku
|
|
Postal code
|
158-8501
|
|
Town
|
Tokyo
|
|
Country
|
Japan
|
|
Organisation
|
National Institute of Health Sciences
|
|
Department
|
Division of Risk Assessment
|
|
Title
|
Dr.
|
|
First name
|
Akihiko
|
|
Last name
|
Hirose
|
|
Address
|
1-18-1 Kamiyoga
|
|
Address
|
Setagaya-ku
|
|
Postal code
|
158-8501
|
|
Town
|
Tokyo
|
|
Country
|
Japan
|