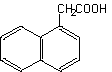
1-Naphthylacetic acid
1-ナフチル酢酸
CAS No. 86-87-3
1-Naphthalene acetic acid
Molecular formula: C12H10O2 Molecular weight: 186.22

In the repeat dose oral toxicity test, body weight gain decreased in both sexes of the 625 mg/kg group. Hematological examination revealed a slight anemia in males of the 625 mg/kg group. Blood chemical examination revealed changes in items related to kidney or liver function in both sexes of the 625 mg/kg group. Relative liver and kidney weights increased and relative spleen weight decreased in males of the 125 or 625 mg/kg groups. Absolute liver weight increased and ovary weights decreased in females of the 125 or 625 mg/kg group. Histopathological examination revealed peripheral fatty change of liver and hepatocellular swelling in females of the 125 and 625 mg/kg groups. The NOEL for repeat dose toxicity is considered to be 25 mg/kg/day for both sexes.
1-Naphthylacetic acid was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA. Although continuous treatment (24 and 48 h) with this chemical substance did not induce chromosomal aberrations in CHL/IU cells, specimens sampled 24 h after the start of 6 h treatment showed marginal structural aberrations. This effect was confirmed by in vitro micronucleus assay. Lowering of the pH of the culture medium may have contributed to the response.
| Purity | : | 99.7% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-day Repeat Dose Toxicity Testing of Chemicals (Japan) |
| Route | : | Oral |
| Dosage | : | 0(vehicle),25,125,625 mg/kg/day |
| Number of animals | : | Male, 5; female, 5 /group |
| Vehicle | : | 0.5% CMC-sodium solution |
| Administration period | : | Male and female, 28 days |
| Terminal kill | : | Male and female, days 29 or 43 |
| GLP | : | Yes |
The NOEL is considered to be 25 mg/kg/day for males and females under the conditions of the present study.
| Purity | : | 96.4% |
| Test species/strains | : | S.typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedures | : | Plate incorporation method |
| Solvent | : | DMSO |
| Positive controls | : | -S9, AF-2 (TA100, WP2, TA98), sodium azide (TA1535) and 9-aminoacridine (TA1537) +S9, 2-aminoanthracene (all strains) |
| Dosage | : | 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plate/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Genetic effects:
S. typhimurium TA100, TA1535, TA 98, TA1537
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 96.4% |
| Type of cell used | : | Chinese hamster CHL/IU cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Dosage | : | -S9 (continuous treatment): 0, 0.3, 0.6, 1.1 mg/ml -S9 (short-term treatment): 0, 0.4, 0.9, 1.7 mg/ml +S9 (short-term treatment): 0, 0.4, 0.9, 1.7 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| with metabolic activation: | [*] | [ ] | [ ] | [ ] | [ ] | [*] |
| 1) | The test was performed by the Biosafety Research Center, Foods, Drugs and Pesticides (An-pyo Center), 582-2 Shioshinden Arahama, Fukude-cho,Iwata-gun, Shizuoka 437-12, Japan. Tel +81-538-58-1266 Fax +81-538-58-1393 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |