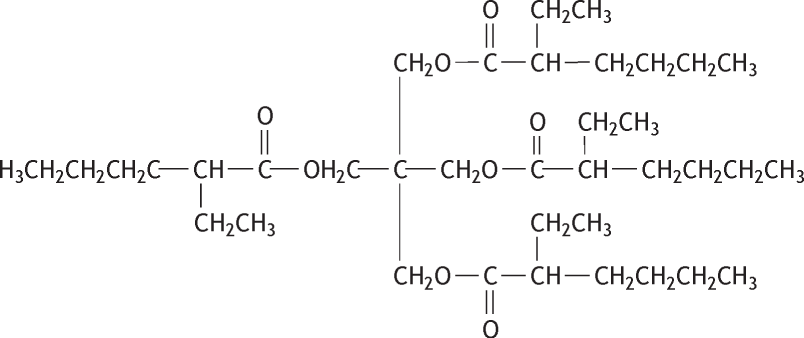
Molecular formula: C37H68O8 Molecular weight: 640.94

Pentaerythritol tetra(2-ethylhexanoate) was studied for oral toxicity in female rats in a single dose toxicity test at doses of 300 and 2000 mg/kg. The chemical was classified into category 5 of the GHS concerning acute toxicity.
A combined repeated dose and reproductive/developmental toxicity screening test of pentaerythritol tetra(2-ethylhexanoate) was conducted in rats according to the OECD Test Guideline 422. Oral administration of the compound at doses of 0, 100, 300 and 1000 mg/kg did not cause death. No changes related to the compound were observed for general condition, body weights or food consumption. No alterations were observed in the hematology, biochemistry, organ weight and pathology data. There were no adverse effects on reproductive ability, including delivery and lactation. Observation of pups revealed no effects of the compound on viability, body weights or morphology. The NOAEL, both for repeated dose toxicity and reproductive and developmental toxicity, is considered to be 1000 mg/kg/day.
Reverse mutation tests using microorganisms (Salmonella typhimurium, Escherichia coli) were conducted to assess the potential of pentaerythritol tetra(2-ethylhexanoate) to induce gene mutations.
Pentaerythritol tetra(2-ethylhexanoate) did not induce gene mutations in bacteria under the conditions of this study.
In vitro chromosomal aberration tests using cultured Chinese hamster cells (CHL/IU) were conducted to assess the potential of pentaerythritol tetra(2-ethylhexanoate) to induce chromosomal aberrations.
Pentaerythritol tetra(2-ethylhexanoate) did not induce chromosomal aberrations in cultured cells under the conditions of this study.
SUMMARIZED DATA FROM THE STUDIES
| Purity | : | 98.4 % |
| Test species/strain | : | Rat/Crj: CD(SD) |
| Test method | : | OECD Test Guideline 423 |
| Route | : | Oral(gavage) |
| Dosage | : | 300 and 2000 mg/kg |
| Number of animals/group | : | Females, 6 |
| Vehicle | : | Corn oil |
| GLP | : | Yes |
Test results:
No deaths were observed with either 300 and 2000 mg/kg during the observation period. Diarrhea was observed in one animal receiving 300 mg/kg on the administration day.
The LD50 value was found to be higher than 2000 mg/kg, therefore the chemical was classified into category 5 of the GHS concerning acute toxicity.
2. Repeated Dose and Reproductive/Developmental Toxicity 2)
| Purity | : | 96.4 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 422 |
| Route | : | Oral(gavage) |
| Dosage | : | 0(vehicle), 100, 300, 1000 mg/kg/day |
| Number of animals/group | : | Males, 12(5 for recovery) Females, 12; satellite females, 5 |
| Vehicle | : | Corn oil |
| Administration period | : | Males, 42 days Females, from 14 days before mating to day 4 of lactation Females(satellite), 42 days |
| Recovary period | : | Males, 14 days Females(satellite), 14 days |
| Terminal killing | : | Males, day 43 of treatment and day 15 of recovery Females, day 5 of lactation Females(satellite), day 15 of recovery Offspring, 4 days after birth |
| GLP | : | Yes |
Test results:
<Repeated dose toxicity and recovery test>
No deaths were found in any groups. No changes related to the compound were observed with regard to general condition, body weights and food consumption during the treatment period. No findings related to the compound were observed in the hematology, biochemistry, organ weight or pathology data at the end of both the dosing and the recovery periods. Detailed examination of clinical signs and functional tests revealed no abnormalities suggestive of neurotoxicity.
<Reproductive and developmental toxicity>
There were no adverse effects on the estrous cycle, mating index, fertility index, gestation index, gestation length, number of corpora lutea, number and rate of implantations, and delivery index. Observations of pups revealed no effects of the compound on viability, sex ratio and body weight. There were no abnormalities in morphology of pups related to dosing.
<No observed adverse effect level(NOAEL)>
From these results, under the conditions of this study, the NOAEL, both for repeated dose toxicity and reproductive and developmental toxicity, is considered to be 1000 mg/kg/day.
3. Genetic Toxicity
3-1. Bacterial test 1)
| Purity | : | 98.4 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Acetone |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA), sodium azide (TA1535) and 9-aminoacridine hydrochloride (TA1537) +S9 mix; 2-Aminoanthracene (all strains) |
| Dosage | : | [Dose-finding study] -S9 mix; 0, 8.19, 20.5, 51.2, 128, 320, 800, 2000, 5000 μg/plate (all strains) +S9 mix; 0, 8.19, 20.5, 51.2, 128, 320, 800, 2000, 5000 μg/plate (all strains) [Main study] -S9 mix; 0, 78.1, 156, 313, 625, 1250, 2500 μg/plate (all strains) +S9 mix; 0, 78.1, 156, 313, 625, 1250, 2500 μg/plate (all strains) [Restudy] -S9 mix; 0, 78.1, 156, 313, 625, 1250, 2500 μg/plate (TA1535) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 3 |
| GLP | : | Yes |
Test results:
No increase in revertant colonies was observed in the test with either the non-activation method (-S9 mix) or the activation method (+S9 mix).
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
3-2. Non-bacterial in vitro test(chromosomal aberration test)1)
| Purity | : | 98.4 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Ethanol |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 1250, 2500, 5000 μg/mL +S9 mix (short-term treatment); 0, 1250, 2500, 5000 μg/mL -S9 mix (continuous treatment); 0, 1250, 2500, 5000 μg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
No increase in chromosomal aberrations was observed with either the short-term treatment (-S9 mix and +S9 mix) or the continuous treatment.
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | : | The tests were performed by the Biosafety Research Center, Foods, Drugs and Pesticides(An-pyo Center), 582-2 Shioshinden, Iwata-shi, Shizuoka, 437-1213, Japan. Tel +81-538-58-1266, Fax +81-538-58-1393 |
| 2) | : | The test was performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751, Fax +81-463-82-9627 |