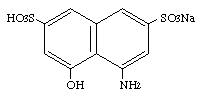

Monosodium 4-amino-5-hydroxy-2,7,-naphthalenedisulfonate was studied for oral toxicity in rats in a single dose toxicity test at a dose of 2000 mg/kg and in a 28-day repeat dose toxicity test at doses of 0, 30, 100, 300 and 1000 mg/kg/day. Monosodium 4-amino-5-hydroxy-2,7,-naphthalenedisulfonate was also tested for mutagenicity with assays for reverse mutation in bacteria and chromosomal aberrations in cultured Chinese hamster (CHL) cells.
The single dose oral toxicity test revealed LD50s of above 2000 mg/kg for both sexes.
In the repeat dose study, the high dose group showed caecum enlargement, without any associated pathological changes. NOEL for repeat dose toxicity was achieved at 1000 mg/kg/day.
Monosodium 4-amino-5-hydroxy-2,7-naphthalenedisulfonate was not mutagenic for S. typhimurium TA100, TA1535, TA98, TA1537 and E. coli WP2 uvrA with or without exogenous metabolic activation up to 5000 μg/plate. This chemical induced neither chromosomal aberrations nor polyploidy with or without exogenous metabolic activation up to the cytotoxic concentration.
| Purity | : | 87.4 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Test Guideline 401 |
| Dose | : | 2000 mg/kg |
| Number of animals | : | Male; 5, Female; 5 |
| GLP | : | Yes |
| Test results | : | LD50: Male, > 2000 mg/kg; Female, > 2000 mg/kg |
| Purity | : | 87.4 % |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | Guidelines for 28-Day Repeat Dose Toxicity Test of Chemicals (Japan) |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 30, 100, 300, 1000 mg/kg/day |
| Number of animals | : | Male, 6; Female, 6/group |
| Vehicle | : | 1 w/v% methylcellulose solution |
| Administration period | : | Male and Female, 28 days |
| Terminal kill | : | Male and Female, days 29 or 43 |
| GLP | : | Yes |
| Purity | : | 87.4% |
| Test species/strains | : | S. typhimurium TA100, TA1535, TA98, TA1537 E. coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Procedure | : | Plate method |
| Solvent | : | DMSO |
| Positive controls | : | -S9, AF-2 (TA100, WP2 uvrA, TA98), sodium azide(TA1535) and 9-aminoacridine (TA1537) +S9, 2-Aminoanthracene (all strains) |
| Doses | : | 0, 312.5, 625, 1250, 2500, 5000 μg/plate |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
Genotoxic effects:
S. typhimurium TA100, TA1535, TA98, TA1537
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
E. coli WP2 uvrA
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 87.4% |
| Type of cell used | : | Chinese hamster CHL cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | 0.5% CMC Na solution |
| Positive controls | : | -S9, Mitomycin C +S9, Cyclophosphamide |
| Doses | : | -S9: 0, 0.55, 1.1, 2.2 mg/ml +S9: 0, 0.85, 1.7, 3.4 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Genotoxic effects:
| + | ? | - | |
| with metabolic activation: | [ ] | [ ] | [*] |
| without metabolic activation: | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Research Institute for Animal Science in Biochemistry and Toxicology, 3-7-11 Hashimotodai, Sagamihara-shi, Kanagawa, 229, Japan. Tel 81-427-62-2775 Fax 81-427-62-7979 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel 81-463-82-4751 Fax 81-463-82-9627 |