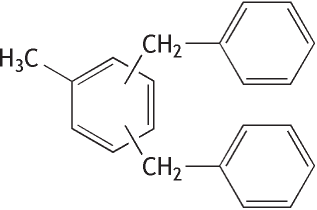
Molecular formula: C21H20 Molecular weight: 272.38

Dibenzyltoluene was orally administered to male and female Crj:CD(SD)IGS rats at dose levels of 0, 20, 100 and 500 mg/kg for 28 days, and its toxicity potential and recovery were examined.
Changes attributable to the test substance were apparent in hematology, organ weight, and histopathological data.
In hematology, prolonged PT and APTT were noted in males receiving 500 mg/kg.
Organ weight measurement revealed increases in liver and kidney weights in males and females receiving 500 mg/kg. In addition, an increase in liver weight was noted in males receiving 100 mg/kg.
At necropsy, enlargement of the liver was noted in males and females receiving 500 mg/kg and histopathological examination revealed centrilobular hypertrophy of the hepatocytes in these animals.
There were no changes related to the test substance for clinical observations, functional tests, body weights, food consumption, blood chemistry or urinalysis.
In summary, significant changes attributable to the test substance were noted at 100 mg/kg or more in males and at 500 mg/kg in females. Therefore, the NOEL is considered to be 20 mg/kg/day for males and 100 mg/kg/day for females from this study.
| Purity | : | 99.62 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 407 |
| Route | : | Oral(gavage) |
| Dosage | : | 0(vehicle), 20, 100, 500 mg/kg/day |
| Number of animals/group | : | Males, 10; females, 10(0, 500 mg/kg) Males, 5; females, 5(20, 100 mg/kg) |
| Vehicle | : | 0.1 % Tween 80 aqueous solution |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Days 29 or 43 |
| GLP | : | Yes |
Test results:
Changes attributable to the test substance were apparent in hematology data and organ weights, and on histopathological examination. In hematology, prolonged PT and APTT were noted in males receiving 500 mg/kg. Organ weight measurement revealed increases in liver and kidney weights in males and females receiving 500 mg/kg. In addition, an increase in liver weight was noted in males receiving 100 mg/kg. At necropsy, enlargement of the liver was noted in males and females receiving 500 mg/kg and histopathological examination revealed centrilobular hypertrophy of the hepatocytes in these animals.
There were no changes related to the test substance for clinical observations, functional tests, body weights, food consumption, blood chemistry or urinalysis.
In summary, significant changes attributable to the test substance were noted at 100 mg/kg or more in males and at 500 mg/kg in females. Therefore, the NOEL is considered to be 20 mg/kg/day for males and 100 mg/kg/day for females from this study.
| 1) | The test was performed by the Kashima Laboratory, Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Kamisu-shi, Ibaraki 314-0255, Japan. Tel +81-479-46-2871, Fax +81-479-46-2874 |