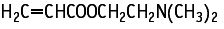
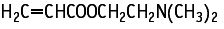
With regard to repeat dose toxicity, two females died, and suppression of body weight gain and decrease in food consumption were observed in males in the 100 mg/kg group. Increased in reticulocyte, platelet and segmented neutrophil counts, and a decrease in albumin were also noted in this group. Histopathological examination revealed ulceration, inflammatory cell infiltration and hyperplasia of the mucosa in the forestomach, and hyperplasia of plasma cells in the pancreatico-duodenal lymph nodes in both sexes, and atrophy of the thymus in females of the same group. In the 20 mg/kg group, similar histopathological changes were observed in the forestomach in males. The NOELs for repeat dose toxicity are considered to be 4 mg/kg/day for males and 20 mg/kg/day for females. In terms of reproductive/developmental toxicity, the compound had no effects on any relevant parameters. The NOELs for reproductive/developmental toxicity are considered to be 100 mg/kg/day for parental animals and offspring.
2-(Dimethylamino)ethyl acrylate was mutagenic in Salmonella typhimurium TA98, with an exogeneous metabolic activation system.
Genotoxicity of 2-(dimethylamino) ethyl acrylate was studied by chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells. Structural chromosomal aberrations were induced with continuous treatment at 0.060 mg/ml (high concentration), and short-term treatment with and without an exogenous metabolic activation system at 0.050 and 0.010 mg/ml (both high concentrations), respectively. Polyploidy was induced with continuous treatment at 0.060 mg/ml, short-term treatment with the metabolic activation system at 0.025 and 0.050 mg/ml (low and high concentrations), and short-term treatment without metabolic activation system at 0.0050 and 0.010 mg/ml (low and high concentrations).
| Purity | : | 99.9% |
| Test species/strain | : | Rats/Crj:CD (SD) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Dosage | : | 0 (Vehicle), 4, 20, 100 mg/kg/day |
| Number of animals | : | Males, 12; females, 12/group |
| Vehicle | : | Corn oil |
| Administration period | : | Males, 43 days Females, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Males, day 44 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
The NOELs for repeat dose toxicity are considered to be 4 mg/kg/day for males and 20 mg/kg/day for females.
The NOELs for reproductive and developmental toxicity are considered to be 100 mg/kg/day for parental animals and offspring.
| Purity | : | 99.9 wt% |
| Test species/strains | : | Salmonella typhimurium, TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD Guideline No. 471 and 472 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Water |
| Positive controls | : | -S9 mix, 2-(2-Furyl)-3-(5-nitro-2-furyl) acrylamide (TA100, TA98, WP2), Sodium azide (TA1535) and 9-Aminoacridine (TA1537) +S9 mix, 2-Aminoanthracene (five strains) |
| Doses | : | -S9 mix; 0, 78.1, 156, 313, 625, 1250, 2500 μg/plate (TA98, TA1537) 0, 156 - 5000 μg/plate (TA100, TA1535, WP2) +S9 mix; 0, 156 - 5000 μg/plate (TA100, TA1535, TA98, TA1537) 0, 313 - 5000 μg/plate (WP2) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA98
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [*] | [ ] | [ ] |
Salmonella typhimurium TA100, TA1535, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.9 wt% |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD Guideline No. 473 |
| Solvent | : | Distilled water |
| Positive controls | : | -S9 mix, Mitomycin C +S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix (continuous treatment):0, 0.015, 0.030, 0.060 mg/ml -S9 mix (short-term treatment):0, 0.0050, 0.010 mg/ml +S9 mix (short-term treatment):0, 0.025, 0.050 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| Without metabolic activation (continuous treatment) | :0.060 mg/ml (clastogenicity) :0.060 mg/ml (polyploidy) |
| Without metabolic activation (short-term treatment) | :0.010 mg/ml (clastogenicity) :0.0050 mg/ml (polyploidy) |
| With metabolic activation (short-term treatment) | :0.050 mg/ml (clastogenicity) :0.025 mg/ml (polyploidy) |
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [*] | [ ] | [ ] | [*] | [ ] | [ ] |
| With metabolic activation: | [*] | [ ] | [ ] | [*] | [ ] | [ ] |
| 1) | The tests were performed by the Mitsubishi Chemical Safety Institute Ltd., 14 Sunayama, Hasaki-machi, Kashima-gun, Ibaraki, 314-02, Japan. Tel +81-479-46-2871 Fax +81-479-46-2874 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |