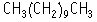
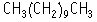
The single dose toxicity test revealed an LD50 value of above 2000 mg/kg for both sexes.
In the repeat dose toxicity test, salivation was observed in males and females given 300 or 1000 mg/kg. Body weight gain was suppressed in males given 1000 mg/kg, and body weights was increased in females given 1000 mg/kg during the lactation period. Food consumption was decreased in males given 300 and 1000 mg/kg in the first half of the administration period, increased in males given 1000 mg/kg in the second half of administration period, and increased in females given 1000 mg/kg in the second half of pregnancy and during the lactation period. Hemoglobin concentration was decreased in males given 1000 mg/kg. Undecane caused liver changes such as increased relative organ weights in males and females given 1000 mg/kg, and a decrease in serum albumin and increases in serum α2-globulin, GPT, cholinesterase and total cholesterol in males given 1000 mg/kg. The NOEL for repeat dose toxicity is considered to be 100 mg/kg/day for both sexes. Body weight gain was decreased in male and female offspring of the 1000 mg/kg group. No effects were observed on reproductive performance in either sex. The NOEL for reproductive and developmental performances is considered to be 300 mg/kg/day.
Undecane was not mutagenic to Salmonella typhimurium TA100, TA98, TA1535, TA1537 and Escherichia coli WP2 uvrA, with or without metabolic activation.
Undecane did not induce structural chromosomal aberrations or polyploidy in CHL/IU cells up to the limit concentration of 10 mM, in the absence or presence of an exogenous metabolic activation system.
| Purity | : | ≧ 99% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Guidelines 401 |
| Doses | : | 0, 500, 1000, 2000 mg/kg |
| Number of animals/group | : | Males, 5 ; females, 5 |
| GLP | : | Yes |
Test results:
| Purity | : | ≧ 99% |
| Test species/strain | : | Rat/Crj:CD (SD) |
| Test method | : | OECD Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test |
| Route | : | Oral (gavage) |
| Doses | : | 0 (vehicle), 100, 300, 1000 mg/kg/day |
| Number of animals/group | : | Males, 12 ; females, 12 |
| Vehicle | : | Olive oil |
| Administration period | : | Males, 46 days
Females, from 14 days before mating to day 3 of lactation |
| Terminal kill | : | Males, day 47 Females, day 4 of lactation |
| GLP | : | Yes |
Test results:
| Purity | : | 99% |
| Test species/strains | : | S.typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test methods | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) and OECD (471 and 472) |
| Procedures | : | Plate incorporation method |
| Solvent | : | Acetone |
| Positive controls | : | -S9 mix, 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, WP2, TA98), Sodium azide (TA1535) and 9-Aminoacridine (TA1537), +S9 mix, 2-Aminoanthracene (five strains) |
| Dosage | : | 0, 313, 625, 1250, 2500 and 5000 μg/plate in five strains, -S9 mix and +S9 mix |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 3 |
| Number of replicates | : | 2 |
| GLP | : | Yes |
Test results:
| + | ? | - | |
| without metabolic activation | [ ] | [ ] | [*] |
| with metabolic activation | [ ] | [ ] | [*] |
| + | ? | - | |
| without metabolic activation | [ ] | [ ] | [*] |
| with metabolic activation | [ ] | [ ] | [*] |
| Purity | : | 99% |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Japan) |
| Solvent | : | Acetone |
| Positive controls | : | -S9 mix, Mitomycin C +S9 mix, Cyclophosphamide |
| Doses | : | -S9 mix (continuous treatment): 0, 0.40, 0.80, 1.6 mg/ml -S9 mix (short-term treatment): 0, 0.40, 0.80, 1.6 mg/ml +S9 mix (short-term treatment): 0, 0.40, 0.80, 1.6 mg/ml |
| S-9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| with metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by the Safety Research Institute for Chemical Compounds Co., Ltd., 363-24, Shin-ei, Toyohira-ku, Sapporo, Hokkaido, 004, Japan. Tel +81-11-885-5031 Fax +81-11-885-5313 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |