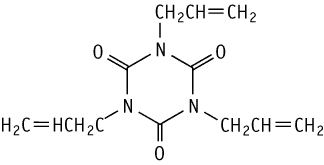

1,3,5-Tris(2-propenyl)isocyanuric acid was studied for oral toxicity in rats in a 28-day repeat dose toxicity test at doses of 0, 5, 15 and 50 mg/kg.
Suppressed body weight gain was observed in males and females of the 50 mg/kg group. Decreased food consumption was observed in males of the 50 mg/kg group. Blood chemistry examination revealed increase in ASAT, ALAT and LDH, and decrease in inorganic phosphorus in males of the 50 mg/kg group, increase in AlP in females of the 50 mg/kg group, and increase in total cholesterol and phospholipid in males and females of the 50 mg/kg group. Pathological examination revealed increase in liver weight in males and females of the 50 mg/kg group and dark red discoloration of the liver in males of the 50 mg/kg group, and histopathological examination revealed hypertrophy of centrilobular hepatocytes in males of the 5 and 15 mg/kg groups and in males and females of the 50 mg/kg group. In addition, single cell necrosis of hepatocytes was observed in males of the 50 mg/kg group, vacuolation of centrilobular hepatocytes in males of the 5 and 50 mg/kg groups, bile thrombi and pericholangitis in males and females of the 50 mg/kg group, and hypertrophy of bile duct cells in males of the 50 mg/kg group.
The NOEL is considered to be lower than 5 mg/kg/day for males and 15 mg/kg/day for females.
Genotoxicity of 1,3,5-tris(2-propenyl)isocyanuric acid was studied by a reverse mutation test in bacteria. This substance was not mutagenic in Salmonella typhimurium TA100, TA98, TA1535, TA1537 or Escherichia coli WP2 uvrA, with or without an exogenous metabolic activation system.
Genotoxicity of 1,3,5-tris(2-propenyl)isocyanuric acid was studied by chromosomal aberration test in cultured Chinese hamster lung (CHL/IU) cells. 1,3,5-Tris(2-propenyl)isocyanuric acid did not induce structural chromosomal aberrations or polyploidy under the present test conditions.
| Purity | : | 99.12 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | OECD Test Guideline 401 |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 250, 500, 1000 mg/kg |
| Number of animals/group | : | Males, 5; females, 5 |
| Vehicle | : | Olive oil |
| GLP | : | Yes |
Test results:
LD50: Male, 707 mg/kg; female, 812 mg/kg
| Purity | : | 99.12 % |
| Test species/strain | : | Rat/Crj:CD(SD)IGS |
| Test method | : | Guideline for 28-Day Repeated Dose Toxicity Test in Mammalian Species (Chemical Substances Control Law of Japan) |
| Route | : | Oral (Gavage) |
| Dosage | : | 0 (Vehicle), 5, 15, 50 mg/kg/day |
| Number of animals/group | : | Males, 6; females, 6 |
| Vehicle | : | Olive oil |
| Administration period | : | Males and females, 28 days |
| Terminal killing | : | Days 29 or 43 |
| GLP | : | Yes |
Test results:
After the recovery period, the changes observed in body weight, food consumption, urinalysis, blood chemistry examination, necropsy as well as single cell necrosis and vacuolation of centrilobular hepatocytes were no longer observed. For the change in organ weight, hypertrophy of centrilobular hepatocytes, bile thrombus, pericholangitis and hypertrophy of bile duct cells, the frequency and degree were reduced, and eosinophilic inclusion bodies and microgranulomas, which reflect restoration, were observed in the centrilobular region. Therefore, reversibility of the changes was suggested.
The NOEL is considered to be lower than 5 mg/kg/day for males and 15 mg/kg/day for females.
| Purity | : | 99.12 % |
| Test species/strains | : | Salmonella typhimurium TA100, TA1535, TA98, TA1537, Escherichia coli WP2 uvrA |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan)and OECD Test Guideline 471 |
| Procedures | : | Pre-incubation method |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; 2-(2-Furyl)-3-(5-nitro-2-furyl)acrylamide (TA100, TA98, WP2 uvrA), Sodium azide (TA1535) and 9-Amino-acridine (TA1537) +S9 mix; 2-Aminoanthracene (five strains) |
| Dosage | : | -S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (five strains) +S9 mix; 0, 156, 313, 625, 1250, 2500, 5000 μg/plate (TA100, TA1535, TA98, TA1537) +S9 mix; 0, 313, 625, 1250, 2500, 5000 μg/plate (WP2 uvrA) |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavon |
| Plates/test | : | 3 (1 for the cytotoxicity test) |
| Number of replicates | : | 2 (plus 1 cytotoxicity test) |
| GLP | : | Yes |
Test results:
Genetic effects:
Salmonella typhimurium TA100, TA98, TA1535, TA1537
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
Escherichia coli WP2 uvrA
| + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] |
| Purity | : | 99.12 % |
| Type of cell used | : | Chinese hamster lung (CHL/IU) cells |
| Test method | : | Guidelines for Screening Mutagenicity Testing of Chemicals (Chemical Substances Control Law of Japan) and OECD Test Guideline 473 |
| Solvent | : | Dimethyl sulfoxide |
| Positive controls | : | -S9 mix; Mitomycin C +S9 mix; Cyclophosphamide |
| Dosage | : | -S9 mix (short-term treatment); 0, 0.63, 1.3, 2.5 mg/mL +S9 mix (short-term treatment); 0, 0.63, 1.3, 2.5 mg/mL -S9 mix (continuous treatment); 0, 0.25, 0.50, 1.0 mg/mL +S9 mix (short-term treatment); 0, 1.5, 2.0, 2.5 mg/mL |
| S9 | : | Rat liver, induced with phenobarbital and 5,6-benzoflavone |
| Plates/test | : | 2 |
| GLP | : | Yes |
Test results:
Genotoxic effects:
| clastogenicity | polyploidy | |||||
| + | ? | - | + | ? | - | |
| Without metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| With metabolic activation: | [ ] | [ ] | [*] | [ ] | [ ] | [*] |
| 1) | The tests were performed by Bozo Research Center Inc, 1284, Kamado, Gotemba-shi, Shizuoka, 412-0039, Japan. Tel +81-550-82-2000 Fax +81-550-82-2379 |
| 2) | The tests were performed by the Hatano Research Institute, Food and Drug Safety Center, 729-5 Ochiai, Hadano-shi, Kanagawa, 257-8523, Japan. Tel +81-463-82-4751 Fax +81-463-82-9627 |